[ad_1]
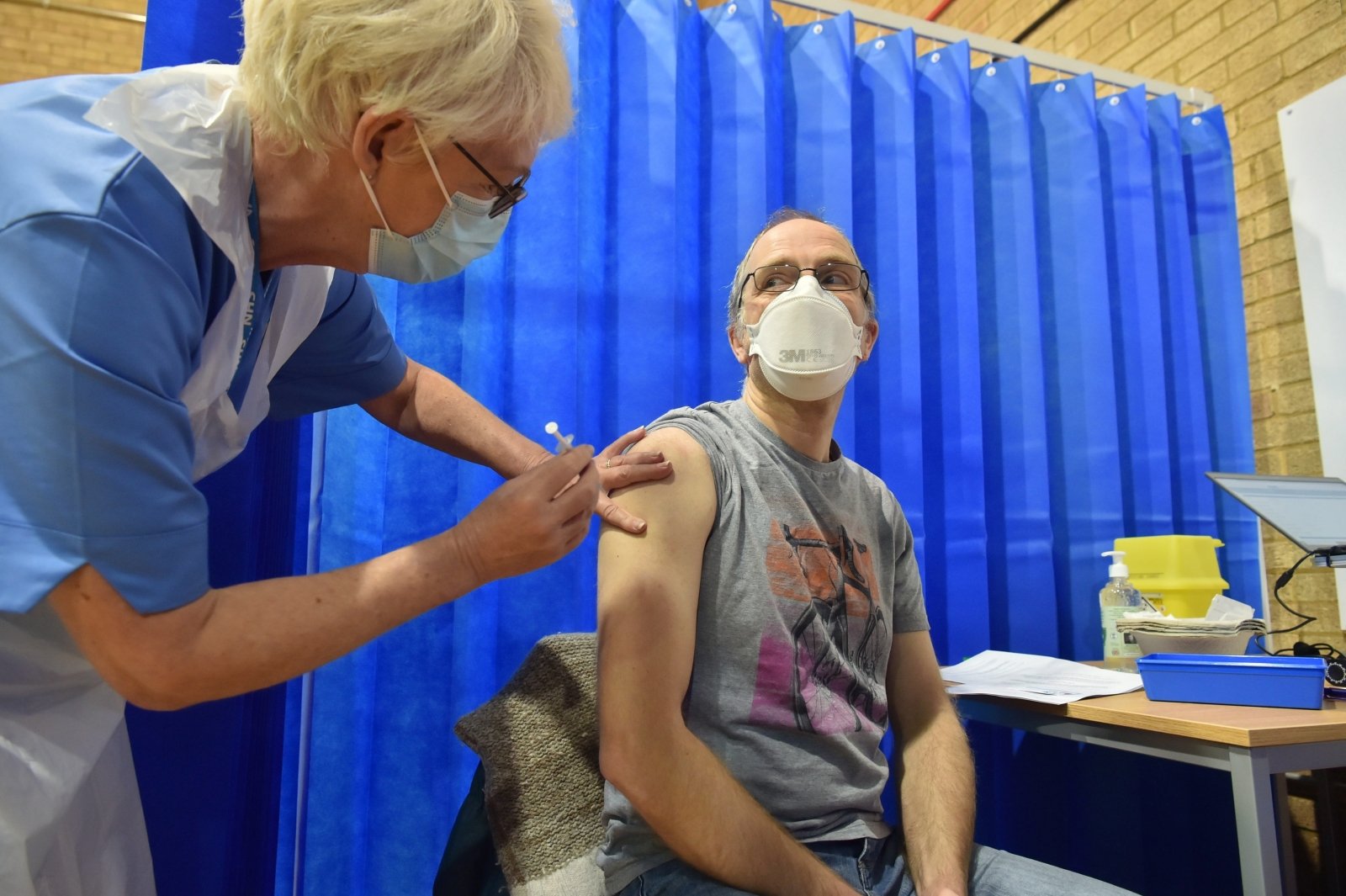
A similar warning has been issued in the United Kingdom (UK), where two healthcare workers have had an allergic reaction to Pfizer-BioNTech.
To date, Pfizer-BioNTech’s two-dose vaccine has only been approved by the UK and Canada, an issue that will be addressed by the FDA’s Advisory Board on Thursday.
“Looking at the data, patients with a history of severe allergic reactions have been excluded from clinical trials,” Slaoui said.
“I am assuming the FDA will make these decisions, that this will be considered and, like the UK, we believe that people with known severe allergic reactions will not be asked to receive this vaccine until we understand exactly what happened here,” he said. .
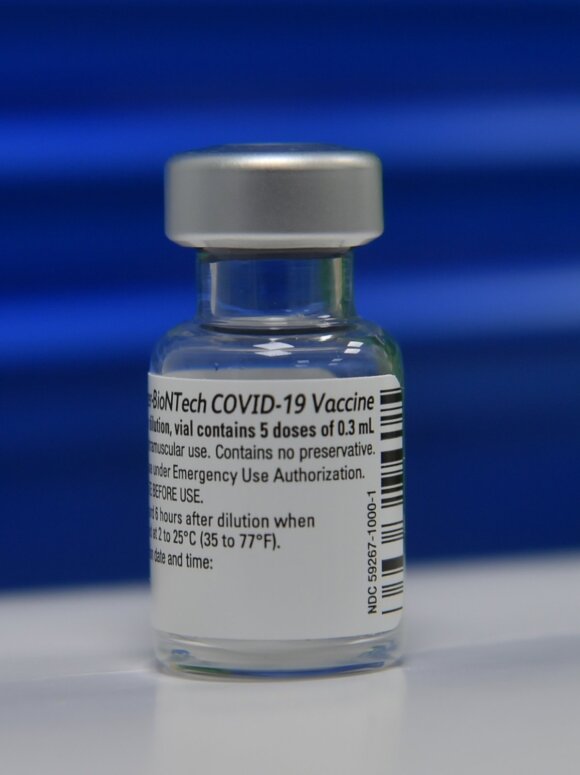
Coronavirus Vaccine with Pfizer
© EPA – ELTA
The FDA is also likely to ask vaccination institutions to be on the lookout for possible side effects, a rare, temporary, and mild form of facial paralysis called Belo’s palsy, as four of the 19,000 people in Pfizer’s clinical trials experienced these side effects.
Mr. Slaoui said he was impressed by the clinical trial data for the vaccine, including the fact that strong immunity to COVID-19 was acquired after the first dose. However, it is recommended that people get vaccinated in two doses three weeks apart.
It is unclear when the FDA will allow the American population to be vaccinated with the Pfizer-BioNTech vaccine, but Health Secretary Alex Azar has hinted that this could be done next week.
General Gus Perna, in charge of the COVID-19 vaccination logistics, said on Wednesday that he had ordered the start of the distribution of syringes, needles, disinfectants and diluents necessary for the vaccination process. The distribution is scheduled to be completed on Friday.
The United States expects to vaccinate 20 million this month. people, especially residents of long-term care facilities and medical personnel. 100 million are expected to be vaccinated by the end of February. people and the entire population of the country in June.
The Moderna, Johnson & Johnson and AstraZeneca vaccines against COVID-19 are expected to be approved after the Pfizer-BioNTech vaccine.
No part of this publication may be reproduced without the written permission of ELTA.
[ad_2]