[ad_1]
Lithuania will purchase a vaccine against COVID-19, the manufacturer of which is not responsible for the side effects caused by the vaccine.
Panic has appeared on the social network Facebook that Lithuania will also buy AstraZeneca vaccines, for whose safety the manufacturer is not responsible.
“But since it is possible to continue after the death of the volunteer, we will test it with complete safety. Funny things. The damn anti-waxes were right after all,” concludes the user of the social network, whose message has been shared more than a thousand times, but don’t jump to hasty conclusions after reading such a message Omits very important information.
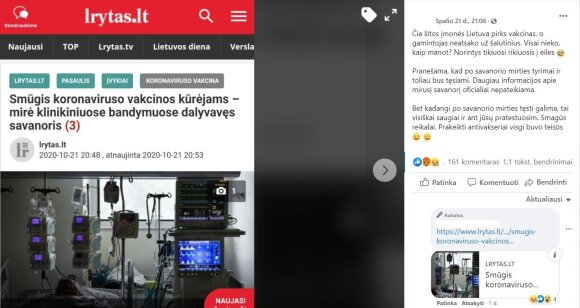
Misleading message
© Screenshot
The death of a 28-year-old Brazilian doctor who worked with COVID-19 patients from complications from the virus was reported in media around the world. Their reports also emphasized that AstraZeneca would not stop testing as a result of the incident, but that did not mean that the death of a volunteer who had been screened for the vaccine had been overlooked.
According to authoritative news outlets such as Forbes, Bloomberg and Reuters, the fact that the vaccine was not stopped means that the deceased volunteer did not receive the vaccine that AstraZeneca is developing.
Half of the study volunteers were vaccinated against the new coronavirus during the trials, and the other half received a safe and licensed meningitis vaccine. A well-known source in Reuters said the vaccine tests would have stopped immediately if the deceased volunteer had been vaccinated against the new coronavirus. As the trials continue, this means that the volunteer was included in a control group that received the meningitis vaccine.
AstraZeneca declined to comment on the incident due to confidentiality and clinical trial rules, but the University of Oxford, which is developing the vaccine with the pharmaceutical giant, confirmed it had no doubts about the safety of the vaccine trials. , and the Brazilian authorities recommended further tests.
The social media user who shared the intimidating message was right that Lithuania was going to buy the vaccine that AstraZeneca was developing. At present, Lithuania has decided and joined the agreements initiated by the European Commission with the vaccine manufacturers AstraZeneca and Johnson & Johnson, and in the future it will decide to buy vaccines from a third company.
Therefore, the author of the message of concern was only partially correct: Lithuania has decided to buy the vaccine that AstraZeneca is developing, but there is no reason to say that this company is not responsible for the side effects of the vaccine.
According to world media, the vaccine tests were not stopped not for lack of responsibility, but because the volunteer was not vaccinated with the vaccine at all. Based on the continuation of the study, it is clear that the deceased man was included in a control group that received a licensed vaccine against meningitis.
- Sources
- Brazil Continues COVID-19 Vaccine Trial Despite Volunteer Death; aljazeera.com
- Covid: No safety issues found with the Oxford vaccine trial after Brazil’s death; bbc.com
- Volunteer dies in Oxford coronavirus vaccine trial, reportedly not receiving experimental vaccine; washingtonpost.com
- Reportedly, the volunteer who died in AstraZeneca’s Covid-19 trial was never vaccinated; forbes.com
- The deceased volunteer in the AstraZeneca trial did not receive the vaccine; bloomberg.com
- A 28-year-old volunteer in AstraZeneca’s coronavirus vaccine trial died, but a report says he was in the control group and received a placebo; businessinsider.com
- Veryga met with representatives of the coronavirus vaccine developers; delfi.lt
It is strictly prohibited to use the information published by DELFI on other websites, in the media or elsewhere, or to distribute our material in any way without consent, and if consent has been obtained, it is necessary to indicate DELFI as the source.
[ad_2]