
[ad_1]
Subsequently, the Lithuanian Ministry of Health (SAM) organized an urgent remote press conference to present Lithuania’s position following the above-mentioned EVA decision.
Health Minister Arūnas Dulkys announced that vaccination with the Vaxzevria vaccine will continue.
“Each drug or procedure has fewer or more side effects, but it is more important to evaluate their overall benefits, look at them holistically. The European Medicines Agency has clearly stated that the benefits of the Vaxzevria vaccine, which outweigh the very rare side effects, outweigh the risks, ”Dulkys said.
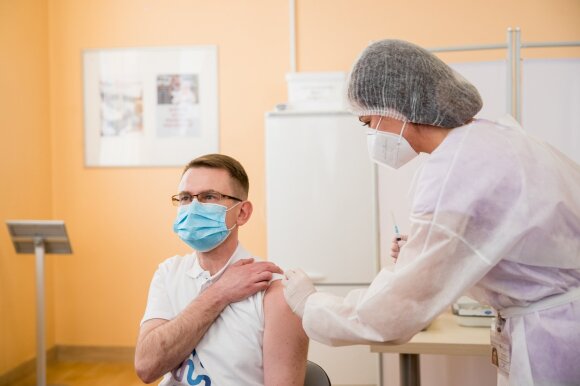
The minister promised that anyone who agreed to be vaccinated with the vaccine would be more informed about possible side effects that should be monitored between 3 and 16 days after vaccination.
“Everyone who agrees to be vaccinated with Vaxzevria will be further informed about symptoms that should be monitored 3-16 days after vaccination,” Dulkys said.
When is it worth worrying about?
Dr. Sabine Straus, chair of the EEA Safety Committee, said earlier: “It is important that healthcare professionals and people who come for vaccination are aware of these risks and monitor the possible symptoms that usually appear during the two first weeks after vaccination. Symptoms include shortness of breath, chest pain, leg swelling, persistent abdominal pain, neurological symptoms including severe or persistent headache, blurred vision, or bruising of the skin at the injection site.

Marius strioga
According to Dr. Marius Strioga, an oncologist at the National Cancer Institute, fever after a vaccine is a normal reaction for the first 2-3 days and should not be exaggerated. If very rare complications occur, they can occur 3 to 16 days after the vaccine. If, on the third day after vaccination, the headache persists, vision and speech deteriorate, the extremities thaw, a medical institution should be consulted as soon as possible. The doctor recommended that you do not wait for all symptoms to appear and consult a doctor if fever, severe pain, and any neurological symptoms begin.
Strioga also explained that there is nothing surprising in the fact that a vaccine can cause side effects, as any medical procedure, such as tooth extraction, can pose a risk.
“It is gratifying that the efforts of scientists have more or less elucidated the possible mechanism that causes thrombocytopenia, which is accompanied by various thromboses. A diagnostic and treatment methodology has already been proposed, ”said Dr. M. Strioga.
According to the oncologist, it is entirely possible to control this condition in time.
“Giving up this vaccine at this stage we are in would be the stupidest thing we could do,” said Dr. M. Strioga.
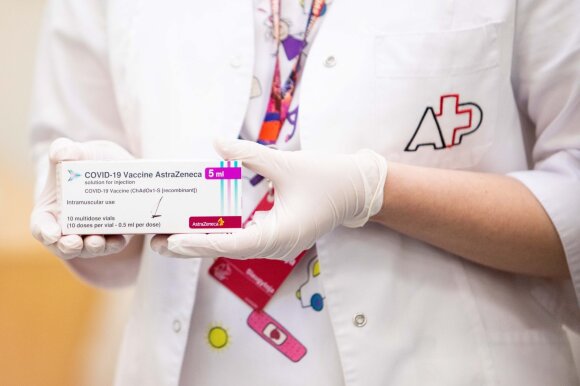
The British offer those under 30 the choice between other vaccines.
However, the vaccine is not recommended for people under 30 years of age in the United Kingdom (United Kingdom). People ages 18 to 29 are offered other vaccinations, the BBC reports.
© DELFI / Josvydas Elinskas
“Adults aged 18-29 who have no comorbidities … should be offered an alternative COVID-19 vaccine and not an AstraZeneca vaccine where an alternative vaccine is available,” Wei Shen Limas from the UK told a new. UK Joint Committee on Vaccination and Immunization Conference.
Despite a British warning, the EVA did not impose new restrictions on the use of the vaccine in people over 18 in a statement issued Wednesday.
Powder: All age groups should be vaccinated.
Dulkys said that all age groups should be vaccinated with this vaccine, as is currently being discussed at a meeting of European health ministers.
Simonas kairys
© DELFI / Josvydas Elinskas
“The different positions promote the noise of the information. The model we have chosen is based on the position of pharmacovigilance so that people can choose, ”said A. Dulkys.
The minister stressed that it is very important that people can choose which vaccine they want to vaccinate.
“There is a pharmacovigilance system and now it is important that all this additional information reaches the vaccination centers and doctors in the near future,” said the head of the SAM.
“Let’s not forget that the key message is that the overall benefits outweigh all the risks,” he said.
“In our case, as Lithuania has a diversified vaccine portfolio and there are currently options for Pfizer, Moderna, Johnson & Johnson arrives in April, the fundamental principle is that we allow options. We allow ourselves to choose a vaccine knowing the complete information provided by experts and specialists, ”said A. Dulkys.
Will write on the brochure

Gytis Andrulionis
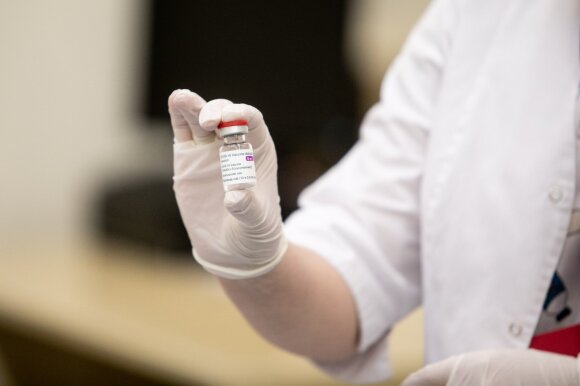
Gytis Andrulionis, director of the State Agency for Drug Control (IARC), noted that it had been decided to include other very rare possible side effects (thrombocytopenia) in the package insert for this vaccine.
“Those very rare side effects can occur 14 days after vaccination. There are approximately 86 cases out of 25 million vaccines “, emphasized G. Andrulionis.
By the way, in Lithuania, according to the head of IWT, the aforementioned side effects did not happen to anyone.
Delfi recalls that the AstraZeneca vaccine against the Vaxzevria coronavirus has returned to the center of attention after Marco Cavaleri, head of the EVA vaccination program, announced on Tuesday that there is a link between thromboembolic events and the vaccine.
© DELFI / Josvydas Elinskas
On Wednesday evening, Amsterdam-based EVA held a virtual press conference in which the agency’s director, Emer Cooke, and other senior officials confirmed that there is such a connection and that it should be seen as a very possible side effect. rare.
Cooke said the EVA had decided, after extensive investigation, that rare cases of blood clots in people who had been vaccinated with AstraZeneca should be included in the list of possible side effects of the vaccine.
At the same time, Cooke added: “Based on the available evidence, specific risk factors such as age, sex or bleeding disorders are not directly related to this.” This risk factor does not depend on age, sex, or pre-existing conditions.
It is strictly forbidden to use the information published by DELFI on other websites, in the media or elsewhere, or to distribute our material in any way without consent, and if consent has been obtained, it is necessary to indicate DELFI as the source.
[ad_2]