[ad_1]
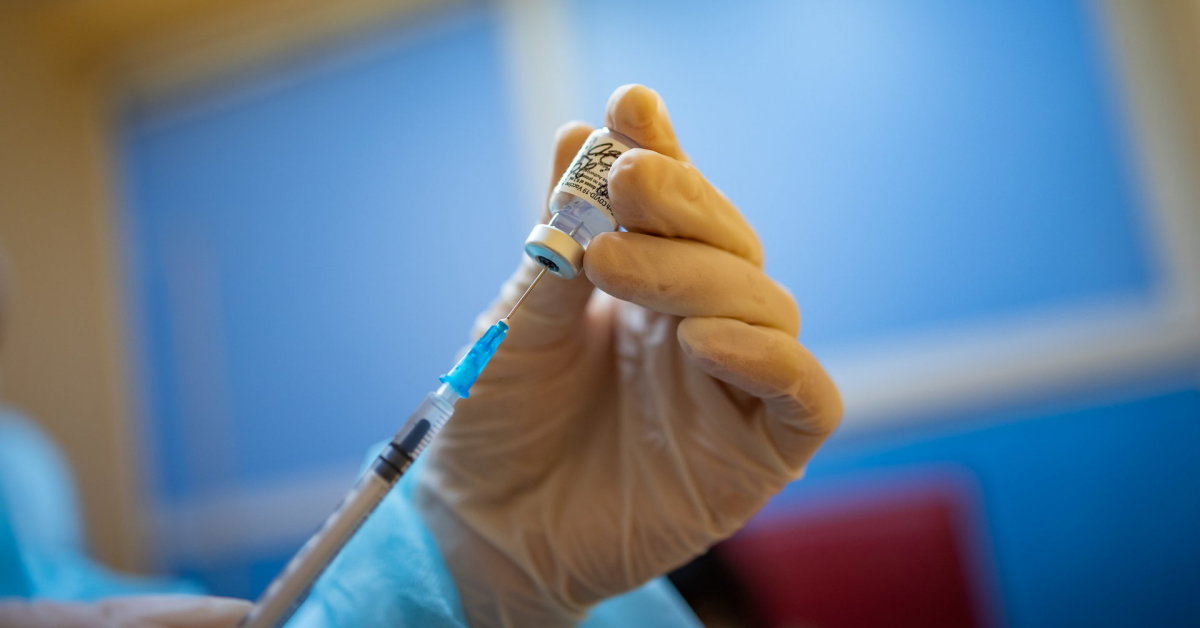
The report said the EEA on Friday recommended that the European Commission (EC) supplement Covid-19 with “Comirnaty “ summary of the characteristics of the product that the reconstituted vaccine can contain 6 doses. An additional condition is specified for the use of syringes and needles that meet certain specifications.
The Ministry of Health informed ASPI about the possible use of the sixth dose. Syringes, needles, and other eligible vaccination kits were shipped with the second batch of vaccine delivered to healthcare facilities. Therefore, treatment facilities can immediately start vaccination with the sixth dose.
According to EVA recommendations, small dose syringes and / or needles should be used to withdraw six doses from one vial. The inactive volume of the combination needle and small inactive syringe should not exceed 35 microliters. If standard syringes and needles are used, the vaccine may not be enough to draw the sixth dose from the vial.
The vaccine should not be drawn from multiple vials to reach the full dose.
If the amount of vaccine remaining in the vial after withdrawing the fifth dose cannot guarantee the full dose (0.3 ml), the healthcare professional should discard the vial and its contents. The vaccine should not be drawn from multiple vials to reach the full dose.
“This morning, the Committee for Medicinal Products for Human Use of the European Medicines Agency, one of the main expert committees, decided to recommend a change to the summary of product characteristics of the medicine.
This is an expert opinion based on the assessment that the vaccine solution has the same consistency, the same effectiveness throughout the container and that the sixth dose is not different from the first or second dose, and that this amount is enough.
That opinion has been submitted to the European Commission and must be approved by the European Commission and, if approved, this amendment will be registered in the Central Register of Medicines and will enter into force throughout the EU from that moment on. ” 15 minutes explained G.Andrulionis, head of the State Drug Control Service (VVKT).
He added that after the EC approved the possibility of using six doses, the same procedure would be established in Lithuania, only secondary technical work would remain. However, it is emphasized that the medical facilities conducting vaccination must have the appropriate equipment.
“Importantly, the EEA opinion, which was provided by experts, indicated that six doses are possible if certain syringes are used.
We have informed the Ministry that the Ministry will evaluate in detail if these syringes are available in the institutions or if they are used. Because if the wrong syringes are used, they can leave a residue (vaccines – red.). As a result, the balance, if it remains high, may not form (sixth dose – ed.) “Said G.Andrulionis.

Photo by Josvydas Elinskas / 15min / Gytis Andrulionis
Aistė Šuksta, a spokesperson for the Health Ministry, said the ministry also has to wait, and the ministry itself will only have to inform medical institutions that they can use six doses after the EC decision takes effect.
“We don’t need to change anything, the tools, the syringes that were delivered to the treatment facilities, meet all the requirements established by the EVA,” he said.
Lithuania has so far only vaccinated the population with coronavirus vaccines made by BioNTech and Pfizer. They are called ‘ice’ vaccines because they must be stored at extremely low temperatures.
To date, five doses of a vaccine from a COVID-19 container have been used. However, both in the world and in Lithuania, specialists disagreed and perhaps the sixth can be used. However, Health Minister Arūnas Dulkys was not ready to support that idea right away: he called for a joint decision from the European Commission.
Outgoing Minister of Health Aurelijus Veryga on his Facebook account He criticized such a decision by A. Dulkis and said that he did not assume responsibility.
“You didn’t take responsibility, you spilled the vaccines. Or maybe not spilled? Maybe it went to someone anyway? Someone not on any of the priority lists?
Lithuania, with the companies BioNTech and Pfizer, has agreed to receive more than 2 million. vaccine dose. More than 40 thousand of them have already been delivered to Lithuania. dose.
[ad_2]