[ad_1]
Easy to do
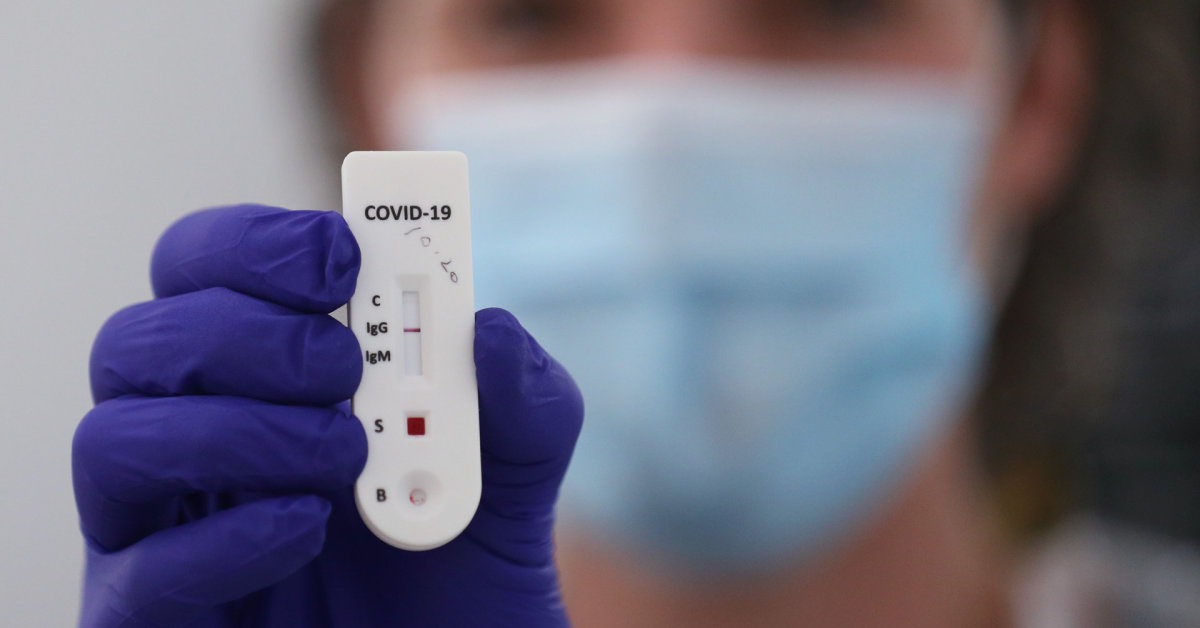
While writing gazeta.pl, Biedronka sells Primacovid serological tests, which reveal whether the person performing the test has COVID-19 IgG and IgM antibodies. According to the sales network, it is easy to use such a test: all you need to do is apply a little blood and in ten minutes there will be a result.
However, keep in mind that these tests do not show whether a person is currently suffering from COVID-19. Only if the infection has reappeared in the past.
Therefore, Polish specialists feared that people who did not fully understand the information would feel safe and this would be a “false sense of security”.

Arno Strumilos / 15min nuotr./ “Biedronka”
Gazeta.pl writes that the price of about 11 euros will be valid until the retailer exhausts the number of tests available. And this is an attractive price, because in private laboratories such a quick test can cost three times as much, more than 30 euros (150 zlotys).
At Biedronka, a customer can buy only three tests at a time.
It is reported that another retail chain in Poland, Lidl, will soon start selling such tests.
He explained how to evaluate the result.
Information also emerged on Monday about how Dr. Maciej Jędrzejko, a physician at the Institute for the Mother and Newborn in Chožov, Silesia, performed this test in front of the camera and explained how to interpret its results.
Scanpix / AP Photo / Antibody test performed
“The test has three bands: IgM, IgC and C, which is a control band. If the latter does not appear, the test failed. If we are healthy and we have not been in contact with the virus, then the control strip will stand out and the other two will not, “explained the doctor, who revealed that he had not acquired resistance to the COVID-19 virus.
He also cautioned once again that this test does not show if you are sick at the moment. However, he assured that the tests are useful, especially knowing that the number of cases in Poland has risen again dramatically. He described the situation as very difficult and said that despite what the country’s authorities are saying, Poland is currently experiencing the same COVID-19 spike.
I can’t sell
So far, no retail chain sells such tests in Lithuanian stores and cannot do so.
According to Ernesta Dapkienė, Director of Maxima’s Image and Communication Department, the network would definitely consider selling such tests.
“As far as we know, the COVID-19 rapid tests currently available on the Lithuanian market are intended for professional use only and not for self-monitoring, as a person cannot use these tests at home without special knowledge.
If the decisions and recommendations of the Ministry of Health (SAM) and other responsible health institutions on the sale of rapid tests for COVID-19 in grocery stores are adopted and the need arises for buyers to purchase rapid tests, we will consider their sale. ”Said E. Dapkienė.
As far as we know, the rapid COVID-19 tests currently available on the Lithuanian market are intended for professional use only and not for self-monitoring, said E. Dapkienė.
Similarly, representatives of other retail chains responded to the question about such a possibility.
Analyze opportunities
Lina Skersytė, public relations representative for Lidl Lietuva, said: “We currently do not have rapid COVID-19 tests for sale, we monitor trends in other countries and analyze the situation and opportunities.”
Renata Keršienė, Director of Public Relations at Rimi Lietuva, stated that “rapid tests for COVID-19 in Rimi chain stores would be a completely new category of products, therefore, before offering it to customers, we must evaluate in a way responsible for legislation and other requirements and ensure that products comply with them. Fully compatible and completely safe. “
We do not have such plans in the near future, because even the regulation is not clear, – said V.Budrienė.
According to Vaida Budrienė, communication manager at UAB Palink, which manages the Iki retail network, this issue should be addressed to SAM, since the sale of such tests should be regulated and only then will the issue be considered by the retail chains.
“We don’t have such plans in the near future, because not even the regulation is clear,” said V. Budrienė.
Darius Ryliškis, a representative from Norfa, also stated that his network does not yet have specific plans.
“The issue is on the agenda”
Advisor to the Minister of Health Aistė Šukšta 15 minutes explained that rapid tests for COVID-19 infection belong to the category of medical devices for in vitro diagnostics: “The supply and distribution of these medical devices is regulated by the Law on the Health System of the Republic of Lithuania and the Union European In Vitro Medical Diagnosis Directive of devices 98/79 / EC.

SAM nuotr./Aistė Šuksta
Rapid tests carried out on the Lithuanian market must be evaluated in accordance with the requirements of that Directive. According to the Accreditation Service, rapid COVID-19 antibody tests and rapid COVID-19 antigen tests distributed in Lithuania are not intended for self-monitoring, when these medical devices can be used by a non-professional person at home, but They are intended for professional use only. “
According to a SAM spokesperson, the results of these tests are evaluated and interpreted by a healthcare professional.
The rapid COVID-19 antibody tests, which are distributed in Lithuania, are not designed for self-monitoring, but only for professional use.
“All medical devices must be used in accordance with the manufacturer’s intended use and in accordance with the manufacturer’s instructions for use. Therefore, the distribution of tests for professional use in pharmacies is not adequate, ”says SAM in a comment.
Currently, the Ministry of Health order stipulates that the examination of a patient using rapid SARS-CoV-2 antigen tests and rapid serological antibody tests has the right to be carried out by all personal health care institutions, except those that provide very specific services.
All COVID-19 disease (coronavirus infection) test orders and responses must be submitted to the Collaborative Infrastructure Information System and Electronic Health Services.
“Based on that, the tests are not for human use. The question of possible self-test validation is on the agenda,” said A.Šukšta.
[ad_2]