[ad_1]
To increase the scope and pace of vaccination, the Ministry of Health is investigating whether Lithuania can independently approve the vaccine and use it without waiting for a decision from the European Commission. And the country’s main hospitals have already begun vaccinating not only doctors but also the first patients against the coronavirus.
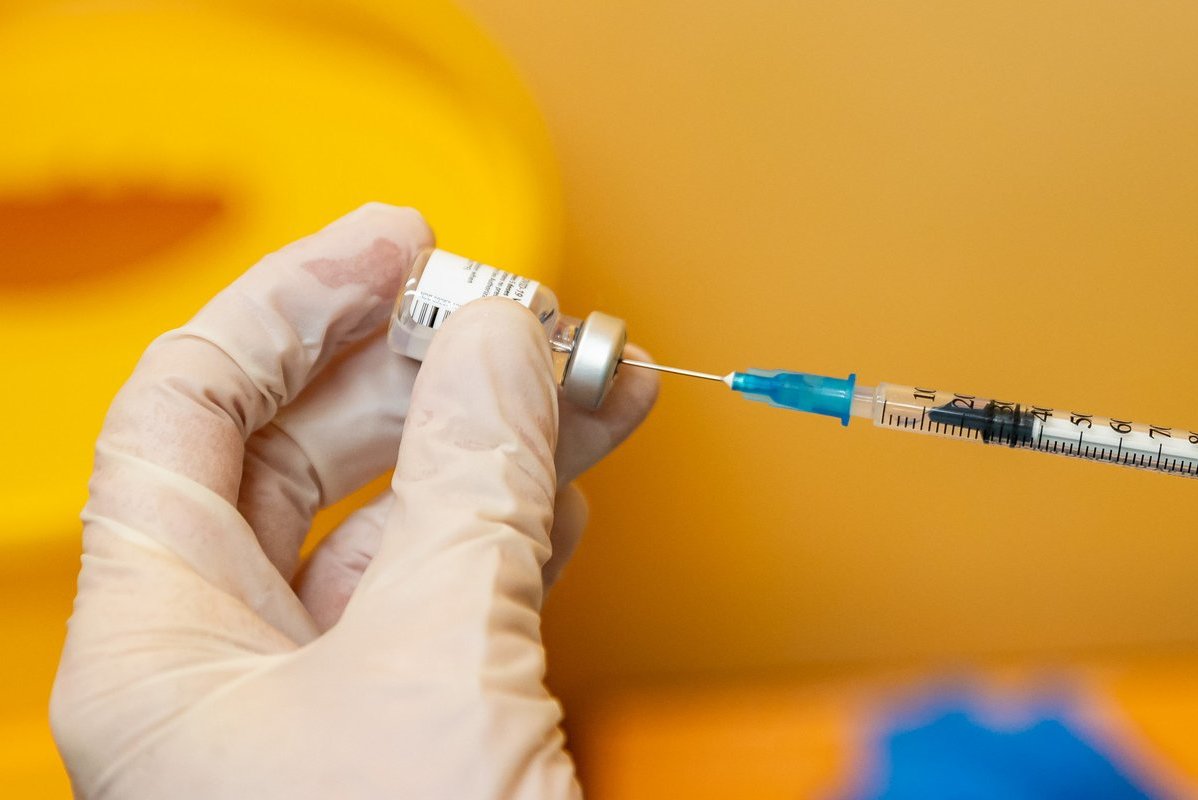
The first batch of coronavirus vaccine from the American company Moderna is expected to arrive in Lithuania next week. Lithuania will receive 496 thousand doses of Moderna vaccine. So far, this is 3.5 times less than the reserved doses of vaccines produced by BioNtech and Pfizer. The number of vaccines arriving in Lithuania next week will not be announced, but it is clear that it will not be high.
“The amount of almost half a million bought by Lithuania extends throughout the entire term of the agreement, which will be much less in January and February, more symbolic,” says Gytis Andrulionis, head of the State Medicines Control.
Moderna is much easier to transport than BioNtech and Pfizer, as it does not require ultra-low temperature freezers. The vaccine will be administered at a temperature of minus 20 degrees. And with it, the neighbors will be vaccinated twice. With an interval of 28 days between the first and second vaccination.
And the country’s major hospitals have already begun vaccinating patients with the third BioNTech and Pfizer vaccine, which was delivered yesterday. More than 300 patients were vaccinated at the Santara clinics and five at the Kaunas clinics.
“The Kaunas clinics have started vaccinating the first patients undergoing chemotherapy. Patients with oncohematological disease were invited. And those who receive transplants are invited, ”says Šarūnas Mačinskas, Head of the Outpatient Services Coordination Service.
It is true that the current quantities of vaccines transported to the country do not satisfy the Ministry of Health. In case of public discontent, the Deputy Minister of Health promised to explain in the Committee for National Security and Defense whether Lithuania could independently confirm other vaccines and start vaccinating more people without waiting for the decision of the European Commission.
“First of all, if it is legally possible, what effect will it have on our joint acquisition agreement with the European Commission or if it will have any consequences. Similarly, if you choose such a method, there would be a real possibility to buy those vaccines, ”says Deputy Minister Živilė Simonaitytė.
According to the Head of the State Drug Control Service, Gytis Andrulionis, according to the Pharmacy Law in force, the Ministry of Health can make the decision to market unregistered drugs. It is true that if Lithuania confirms the vaccine itself, it assumes all risks and responsibility for possible damages.
“We don’t have complete information on the safety, efficacy and all the other data on vaccines. When a pharmaceutical campaign requests registration, they provide all of that data in a very orderly way. And in this case, we have minimal data and some trial data. clinical trials remain confidential, ”said G. Andrulionis.
There is more debate about the increased use of single vial doses of vaccine. Currently, 5 people are vaccinated after diluting one vial of vaccine.
The former Minister of Health, Aurelius Veryga, argues that the practice of other countries that vaccinate 6 or even 7 people against the coronavirus should be used.
“If we said five doses of that dilution were good, why does that sixth all of a sudden become out of place or inappropriate? Why should it be discarded? It is the Minister who has every opportunity to do so and I suggest that he not expect anything, ”says former Minister of Health Aurelijus Veryga.
The Health Ministry has not yet allowed hospitals to vaccinate more than five people with the same vial of vaccine.
“The European Medicines Agency has serious doubts whether this can be done for a variety of things, including the tools that are used. We do not rule out such an opportunity, but first of all we must ensure people’s safety,” says Ž. Simonaitytė .
And while private clinics receive thousands of consultations, people wonder when they will be able to get privately vaccinated against the coronavirus. After all, COVID-19 currently prioritizes vaccines for front-line physicians, high-risk patients, and volunteers who will have worked in a hospital for at least 16 hours in two weeks, and social care centers and of nursing.
“Not only do priority groups want to be vaccinated, every day we receive requests from companies, organizations and individuals about the desire to be vaccinated. But, as we know, now the priority groups are vaccinated and the states buy all the vaccines centrally, ”says Vitalijus Orlovas, CEO of Affidea Lietuva.
Prime Minister Ingrida Šimonytė said today that discussions are underway among health experts on the development of an immune vaccine passport. Such a passport could be obtained by people who have already been vaccinated or infected with coronavirus and have immunity, since this, according to experts, remains for about half a year. And for vaccine passport holders, quarantine requirements would be relaxed.
“It could be one of the ideas that would simplify the quarantine requirements for a part of society. Because now that the quarantine, which has already turned into a fight, has become tougher, we had to listen to why they should be subject to them. restrictions, ”said I. Šimonytė.
Even today, the government changed the pre-purchase agreement with the pharmaceutical company BioNTech and Pfizer. Lithuania is also looking to buy an additional 550,000 doses of coronavirus vaccine. However, the exact number will only be clarified at the end of the negotiations.
[ad_2]