
[ad_1]
The vaccine can be given to anyone over the age of 16, per emergency clearance from the US Food and Drug Administration.The Advisory Committee on Immunization Practices recommendation also states that the vaccine is safe for nearly everyone.
An Alaskan health worker, who has never had an allergic reaction to any vaccine, experienced some pretty side effects after injecting COVID-19 on Tuesday, but health experts say those cases are extremely rare.
“Based on the data that we had access to, these vaccines are very well tolerated,” says Purvi Parikh, an allergist with the Allergy & Asthma Network and one of the vaccine’s trial evaluators.
“We are facing a life-threatening pandemic. We have no way to defend ourselves. Most people should get vaccinated, only in very rare cases could it be argued that it would be better not to vaccinate someone,” says the expert.
Here’s what we already know about who should and shouldn’t get vaccinated against COVID-19.
People allergic to the vaccine.
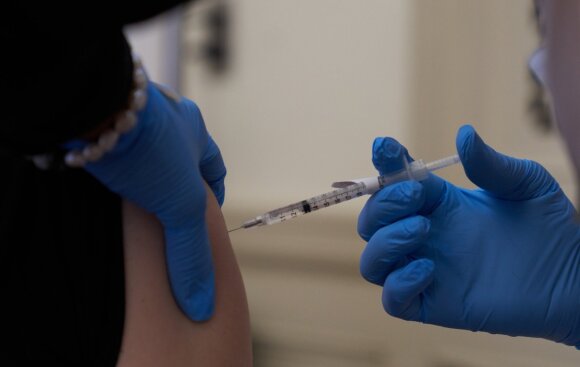
The US Federal Food and Drug Administration has included only one group in the category of those who should not be vaccinated: those who have experienced a severe allergic reaction to any of the ingredients in the Pfizer / BioNTech vaccine.
Vaccine for COVID-19
The warning on the vaccine’s label adds that medical centers must make decisions about how to treat allergic reactions as soon as possible.
Two UK healthcare workers who had experienced allergic reactions to the vaccine in the past experienced side effects within minutes of injecting the COVID-19 vaccine. Both employees have recovered and are feeling great, according to England’s National Health System.
In the UK, people who have had an allergic reaction to the vaccine have not yet been vaccinated against COVID-19. In the United States, the Centers for Disease Control and Prevention states that people with such problems in the past can receive the COVID-19 vaccine. Only these patients should be given all the necessary information about the potential risks. According to Mr. Parikh, they are also advised, although not required, to consult their doctor.
“Your doctor should review your medical history and assess whether or not there is a risk of an allergic reaction. This evaluation is necessary for a very small number of people, a very small number ”, says the expert.
Patients who have previously had an allergic reaction to the vaccine should be monitored for at least half an hour after vaccination, according to the Federal Food and Drug Administration guidelines. For those without a history of allergic reactions, fifteen minutes is sufficient.
People with other allergies
People with other allergies, such as those who are allergic to food or fungi, shouldn’t have a problem with COVID-19.
Vaccine for coronavirus
“In clinical trials, we also included people with allergies, even people with very severe allergies to one or another food. We do not include only those who had an allergic reaction at the time. So there were thousands of people with allergies and the vaccine didn’t cause them any problems, ”says Parikh.
Pregnant and lactating women
Because no data is yet available on the safety of the COVID-19 vaccine in pregnant women, it is up to them to decide if they want to be vaccinated, says Peter Marx, Ph.D., director of the Federal Administration’s Center for Biological Research and Assessment. of Food and Medicines.
“COVID-19 is not good for a pregnant woman, so there may be people who want to get vaccinated, but we cannot strongly recommend it. In this case, the decision is made only by the person himself,” Marks said during a press conference. .
There were no pregnant women in the Pfizer clinical trials, but 23 volunteers became pregnant during the trials. There were no problems. Pfizer / BioNTech says it will continue to monitor these women.
Based on observational data, the absolute risk for pregnant women is identified as low. Because mRNA vaccines do not contain live viruses, they break down quickly in the body and do not enter the cell nucleus. Such a vaccine does not cause genetic changes.
The American Association of Obstetricians and Gynecologists emphasizes that “it would not be worthwhile to deprive pregnant women who meet the vaccination criteria.”
There were no breastfeeding women in clinical trials, but mRNA vaccines should not pose a risk to the breastfed baby, experts say, and the association believes the vaccine should also be offered to breastfeeding women.
Elderly and comorbidities
People with comorbidities and minors should get vaccinated against COVID-19.
Vaccine for COVID-19
The final phase of the Pfizer vaccine trials showed that vaccinating people with comorbidities was as effective as vaccinating healthy people. Older people who participated in the vaccine trials experienced fewer and milder side effects, the company said in a statement.
People with immunosuppression
This vaccine is also suitable for people who have a suppressed immune response due to one disease or another (for example, people with cancer diseases). According to the Federal Food and Drug Administration, this should be a human decision.
Clinical trials of the Pfizer vaccine have been conducted in HIV-infected people with weakened immune systems, but no specific data are available for these people and no conclusions should be drawn regarding the safety of this vaccine in these patients.
“When it comes to pregnant women and those whose immune systems are suppressed, it is very important to look at each case individually,” said Marx.
People with or without COVID-19
Coronavirus vaccine
Late clinical data suggests that the vaccine is safe and helps prevent re-infection with COVID-19. This statement is valid regardless of the severity of the contraction of the new coronavirus by a particular individual.
A person currently suffering from COVID-19 should wait for recovery and only then be vaccinated. There are no recommendations on when the vaccine can be given in case of illness.
People treated with COVID-19 antibodies
There are no safety data in humans treated with antibody therapy or plasma from recovered individuals and the vaccine.
Because reinfection with the new coronavirus is virtually impossible within 90 days of the initial infection, the US Centers for Disease Control and Prevention recommends that the sick person wait at least three months before vaccination.
There is also no evidence that the vaccine can protect a person who has recently been in contact with the infection. People are only protected against the virus if it has been a week or two since the second vaccine.
Teenagers
Vaccine for COVID-19
Adolescents 16 to 17 years old can be vaccinated if they have received the consent of an adult.
The Pfizer vaccine was tested in 153 teens ages 16 and 17, and a preliminary analysis of the data revealed no concerns about the vaccine’s safety.
There is very little detailed information on how this age group responded to the vaccine, but the US Centers for Disease Control and Prevention say that “there is no biological reason to doubt that the vaccine poses any risk for persons under the age of eighteen “.
It is strictly prohibited to use the information published by DELFI on other websites, in the media or elsewhere, or to distribute our material in any way without consent, and if consent has been obtained, it is necessary to indicate DELFI as the source. .
[ad_2]