
[ad_1]
One of my first steps for NVSPL was to reschedule the purchase so that the number of tools purchased was significantly higher than over a period of several months, as was the case before. This was necessary to have a larger stock and to allow more time for other non-high voltage procurement procedures to be carried out, to avoid the need to count out-of-stock tube stocks in the warehouse. We have already done so and we expect the results of these acquisitions by the end of the year or early next.
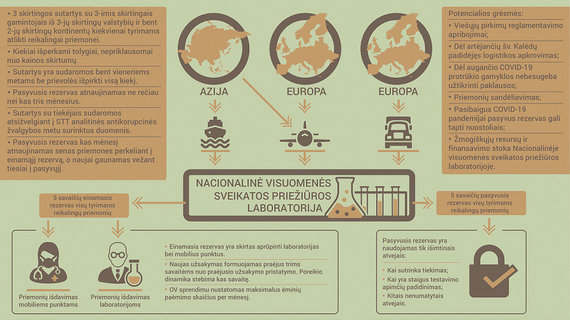
Although NVSPL’s experience so far is not extensive, it is already possible to assess and observe a number of risks in addition to lengthy procurement procedures and insufficient stock of purchased equipment due to the increasing need for investigative tools. As a result, I also looked for more solutions to ensure the sustainable and reliable provision of the necessary tools for COVID-19 investigation. For example, in a more complex epidemiological situation in a country, you may have legislation that prohibits the export of pandemic measures or there is a risk that the plant from which the supplier receives the products will no longer be able to produce them and then deliver them. With the high prevalence of coronavirus across the continent or in one part of the world, action may be impossible; We probably all remember how we continued to transport personal protection and reagents to Lithuania and what competition there was in the world for their acquisition. Not long ago, we also faced a situation where the winner of a public procurement could no longer bring the media to Lithuania and refused to enter into a contract.
NVSPL nuotr./Schema
I think for risk management it would be worthwhile to divide vendors geographically, enter into pre-purchase agreements and use other risk management tools that I present in the box, and here I present a completely new idea to eliminate or at least significantly reduce those risks.
To date, an acquisition is purchased from a specific supplier in an acquisition. What if the supplier could no longer meet its obligations or would it not make more sense to buy from several suppliers at the same time? And to further reduce the risk, I propose to acquire in 3 different contracts with 3 different manufacturers from 3 different countries and at least 2 different continents for each research tool (borrowing this model could also be used successfully in other countries). both through the purchase of personal protective equipment and other measures necessary to manage the pandemic). Then it would be possible to buy instruments uniformly from all 3 states and even in the case of unfavorable situations in one or more of them, we could still obtain the necessary instruments from a third party. It is important to mention that contracts can be concluded for a longer period, such as one year, but without committing to redeem the total maximum amount, that is, there would be no obligation to redeem the full amount of the contract if the situation changes and demand decreases.
In the course of such an acquisition, the information gathered during STT’s intelligence analysis on potential threats to certain legal entities could be used to identify additional risks in advance and to assess which vendors may not be able to meet their obligations.
It would be important to always have a current reserve of around 5 weeks, from which equipment would be constantly supplied to mobile research points and laboratories. Demand dynamics should be monitored regularly, at least once a week. A new order can be formed 2-3 weeks after the previous order is delivered. For smoother maintenance of the system, plans would include the implementation of the KANBAN system, which is necessary for a clear definition and calculation of the process.
It is also necessary to have a passive reserve for a period of at least 5 weeks, which would be used in exceptional cases (for example, in case of interruption, interruption of supply, sudden increase in test volumes or other unforeseen circumstances). The duration of the measures must also be monitored and the measures must be changed regularly, in time to travel to the current reserve to be used.
Unfortunately, with the current legal framework, the implementation of this new concept remains difficult. You also need to address a number of technical issues related to storage, etc. in the development of this system. Therefore, this requires the consensus of various institutions and relevant legislation. Unfortunately, NVSPL alone cannot implement this concept, which requires cooperation. I am pleased that other institutions that can contribute to this are already and are actively involved in the debate on the sustainable delivery of COVID-19 measures. We cannot think much, if we wait, the virus will defeat us. So I hope that the institutions will work together to find ways to address regulatory issues and other nuances, so that in the future all of this is not just in pictures and diagrams, but can really serve the people.
This plan could be applied not only in the fight against a coronavirus pandemic, but also in other global emergencies. Hopefully, there won’t be more, but if something unforeseen happens, it’s always wise to be prepared in advance.
Dan Bakša is the director of the National Public Health Laboratory (NSPL)
[ad_2]