[ad_1]
Starting Tuesday, called V-Day, the first patients over the age of 80 will be vaccinated, including nursing home workers and health and social care workers most at risk of coronavirus infection.
After the first dose of the vaccine, a second vaccine will be needed 21 days later.
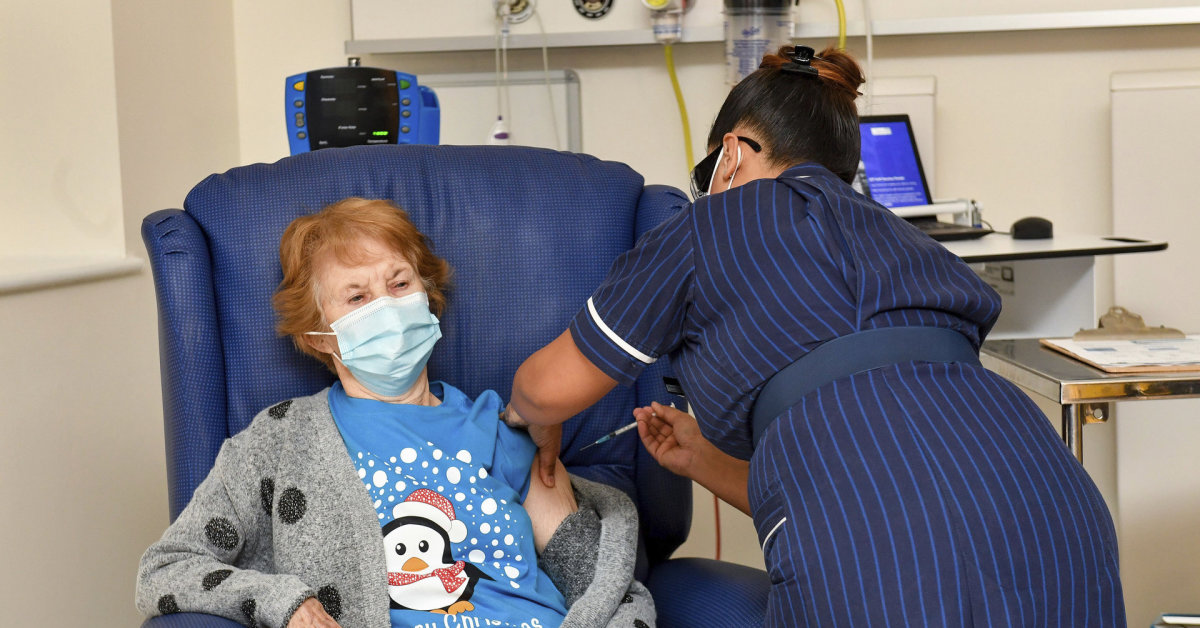
The first person to be vaccinated in Britain on Tuesday morning was Margaret Keenan, 90, of Coventry in central England.
The woman, who wears a penguin-print T-shirt and a gray sweater and protective mask and will turn 91 next week, called the vaccine “the best anticipated birthday gift.”
“My advice to anyone who is offered the vaccine is to get vaccinated. If I can get vaccinated when I am 90 years old, you can too, “he added.
The next shot in line was an old man named William Shakespeare.
Last week, the United Kingdom became the first country to allow the use of a coronavirus vaccine developed by the US pharmaceutical giant Pfizer and the German company BioNTech. This has fueled hopes of a possible breakthrough in the attempt to curb a pandemic that has already cost more than 1.5 million people worldwide. lives.
The United Kingdom is one of the countries most affected in the world by a pandemic. Since the COVID-19 outbreak, 1.6 million cases of infection, more than 61 thousand. people have died from this disease.
Prime Minister Boris Johnson, who spent three nights in the intensive care unit earlier this year with COVID-19, called the launch of the vaccination program “a major step forward in the UK’s fight against coronavirus.”
Health Secretary Matt Hancock, who volunteered to be vaccinated live on television to allay public concern about the safety of the new drug, said the start of vaccination was an “important moment” to protect the most vulnerable.
Simon Stevens, head of England’s National Health Service (NHS), said it was a “crucial turning point” in tackling the “biggest health challenge” since the NHS was founded in 1948.
It has rushed to set up dozens of vaccination centers across the country since the UK supervisory authority turned on the green light to use the vaccine last Wednesday.
Britain has asked for 40 million. dose of 20 million. people. The first batch consists of 800 thousand. dose of vaccine.
It is expected to receive up to 4 million by the end of December. dose of vaccine.
The Queen can lead by example
The mass vaccination program is a coordinated response to the pandemic in the four areas of the United Kingdom: England, Scotland, Wales and Northern Ireland. Typically, these areas determine their own health policies.
The public has widely welcomed the news of the rapid approval of the vaccine, but ministers and health professionals understand that they still have a long way to go to dispel people’s mistrust.
The Independent Medicines and Health Products Regulatory Agency (MHRA) states that the vaccine developed by pharmaceutical companies Pfizer and BioNTech “took no shortcuts” and that the approval procedure for this product met strict international requirements.
The British branch of the NHS said thousands of people had been injected with the vaccine in clinical trials and had not experienced any side effects.
It is reported that Elizabeth II, the 94-year-old British monarch, who owns her vaccine in the early stages of vaccination due to her honorable age, may contribute to a public awareness campaign urging people to get vaccinated.
The government said people would receive vaccination cards to remind them to get vaccinated again in three weeks. However, he stressed that no certificate of immunity is being introduced.
“Mild effect” in winter
Top health officials in England, Scotland, Wales and Northern Ireland said vaccination would have only a “minor effect” on the number of patients in hospitals during the winter months.
Johnson urged people to be patient and strictly adhere to social distancing to avoid increased morbidity, especially since more lenient requirements apply during the Christmas period.
Health officials have already faced logistical challenges in deciding how to vaccinate elderly or disabled nursing home residents.
The vaccine must be stored below -70 degrees Celsius; only hospitals and other medical centers can provide such storage conditions.
Pfizer and BioNTech are being developed in Belgium, so there are concerns about possible supply disruptions when the UK leaves the single market and the EU customs union.
But the UK government says the military is ready to blow up the vaccine if there are problems at the border after January 1.
A large part of the demand for vaccines in Britain should be met by a vaccine developed by the University of Oxford with AstraZeneca, pending approval from the UK drug regulatory authority.
The government has already ordered 100 million. dosage of this preparation. This vaccine is cheaper to produce, easier to store, and can be transported in simple refrigerated vehicles.
[ad_2]