[ad_1]
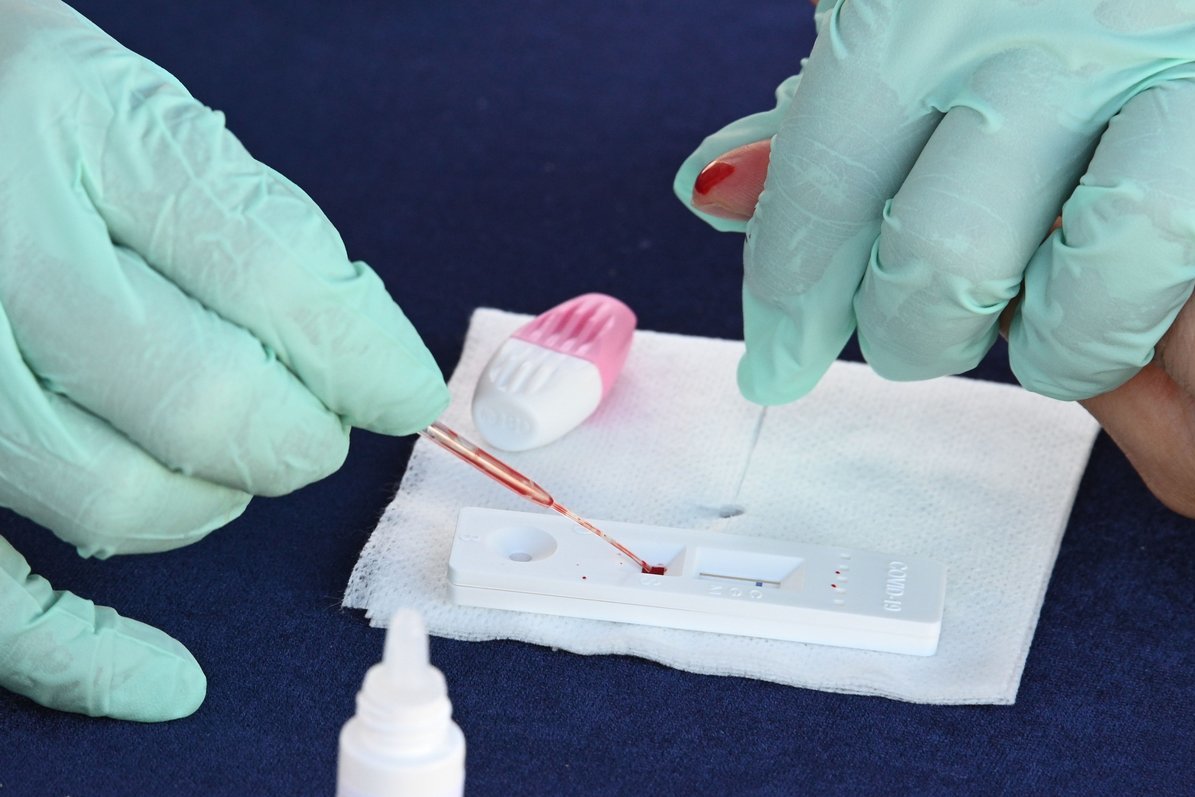
Over € 6 million: both NVSPLs paid Profarma for just over 500 thousand. rapid tests valid until March 2022. From the purchase in March of this year to the date, as announced by NVSPL, of 500 thousand. only 20 thousand tests were used.
NVSPL representatives say they have all been sent to healthcare facilities, with the rest promising to do the same.
Mindaugas Stankūnas, a professor and epidemiologist at the Lithuanian University of Health Sciences, says they are not suitable for diagnosis, so it is difficult to understand why they were bought so much.
“It is very difficult for me to find arguments for who needs them so much.
Perhaps there was some discussion between the Ministry of Health and the National Public Health Laboratory about its use. I don’t want to believe that the decision to buy them was spontaneous and without a clear vision of their use.
Because they are not suitable for diagnosis, their help cannot tell whether a person is suffering from coronavirus or not. They are used to find out if a person is sick and if antibodies have formed. I really don’t see much logic in that, which required half a million rapid tests, M. Stankūnas shares his opinion.
When asked by the professor if the researchers were consulted during the procurement coordination for the purchase of rapid tests, he says that neither he nor, to my knowledge, nor his colleagues at LSMU discussed how many such tests are needed and for whom will be used.
“I did not participate in any discussion about the possibility of acquiring or using these tests. I don’t know that any of the team members in this study (population study) were involved in this discussion. Therefore, I cannot comment on its acquisition history. I don’t know whose decision to buy rapid tests was, “says M. Stankūnas.
There are ideas to use them and more
The professor says he knows the current population study will be done with rapid tests and notes that in an informal setting with colleagues he is considering using these tests for broader research, but so far it is just language.
“There has been a debate among researchers and practitioners that these tests should cover not only randomly selected members of the public, but also certain groups, such as healthcare workers. It would be particularly interesting to consult doctors who have worked with people with coronavirus, as studies in other countries have shown that about 25 percent of medical personnel who have worked with people with coronavirus are already sick.
Meanwhile, the numbers from population surveys are 5-6 times lower.
In Lithuania, we have about 13 thousand doctors, nurses, I think, about 25 thousand. That is, if we try them all, there are actually about 400 thousand left. of those tests, it goes without saying that at least some of them will not be used, ”says M. Stankūnas.
Undeservedly nailed to the cross
In the public sphere, after the purchase of rapid coronavirus tests, it was believed that these tests were not suitable for diagnosis and therefore of little use. However, prof. M. Stankūnas cautions that they are undeservedly nailed to the cross, as a high sensitivity of the tests was determined during the validation.
“True, I must say that the tests themselves are undeservedly hammered on the cross and so far I have no reason to criticize or question these quick tests. Their reliability was verified by scientists at the Vilnius University Life Sciences Center and they were found to be very sensitive and specific, so I believe they are suitable for population research.
I am also aware that these tests are already being used in seroepidemiological studies in other countries of the European Union, such as Croatia ”, says the professor.
In the investigation of rapid test transactions, suspicions
The news portal tv3.lt recalls that on Monday, in the investigation of the FNTT on the purchase of rapid coronavirus tests, suspicions were raised against the representatives of Profarma and the Deputy Minister of Health Lina Jaruševičienė.
The Deputy Minister immediately afterwards submitted a request to resign, and Minister Aurelijus Veryga, as announced by the Ministry representatives, agrees to comply with the request.
The news portal tv3.lt announced that the investigation was announced in early June, as announced, in view of officials: 6 million. Acquisition of rapid coronavirus tests by the National Public Health Laboratory for a value of EUR 1 000 000.

Asked about possible suspicions of the laboratory, Mindaugas Petrauskas, deputy director of the Financial Crime Investigation Service (FNTT), stressed that the head of the laboratory, Vytautas Zimnickas, had died a few weeks ago, and that other employees had not yet been suspected.
It also noted that Ms Jaruševičienė was “the only one among the officials so far” to whom the accusations were made.
The head of the FNTT pointed out that this service is conducting an investigation on rapid tests, while the Special Investigation Service is conducting a separate investigation on the purchase of reagents by the National Public Health Laboratory. These studies, he said, exchange information.
The FNTT investigation was announced in early June, with 6 million people in sight. Acquisition of rapid coronavirus tests by the National Public Health Laboratory for a value of EUR 1 000 000.
According to the FNTT, the pre-trial investigation began after receiving information about the company’s alleged financial transactions.
The funds that Profarma tried to dispose of were obtained after a public purchase contract with the National Public Health Laboratory for the sale of rapid COVID-19 tests. According to the FNTT, the disposition of illegally obtained funds is restricted.
According to the study, which amounts to 6 million. The company allegedly falsified the documents and provided the buyer with false information about the manufacturer of the rapid tests for COVID-19.
The buyer is suspected of possibly being misled by failing to conduct a market investigation and failing to assess the price and market conditions of the purchased goods prior to concluding the contract. After concluding the contract, the funds were transferred to the test provider.
[ad_2]


