[ad_1]
Updated 17 minutes ago
PLANS FOR the deployment of Covid-19 vaccines in Ireland have been released this afternoon, with details on logistics and who will carry out the inoculations included.
The vaccines will be delivered in three phases: the initial launch, a massive surge, and open access. Once a large number of doses are available, the bulking stage can begin.
Highest priority groups: those over 65 in long-term care facilities; and front-line healthcare workers in direct contact with the patient will receive vaccines first. The prioritization strategy of who gets the vaccine in what order was outlined by the government last week.
In announcing the plans, Health Minister Donnelly said: “Today is a really positive day for all of us. After a very difficult year, we are hopeful that Covid-19 vaccines will be approved for us in Ireland in a matter of weeks.
The scale of the Covid-19 vaccination program will be larger and more complex than previous vaccination programs. It will play a central role in our exit from the pandemic. Over time, it will allow us to come back to reopen our society and reconnect in the way we once took for granted.
The minister also said that the first vaccines could be given to people in Ireland before the end of the year, pending approval of the Pfizer / BioNTech vaccine by European authorities.
The vaccines will be administered from long-term care centers, hospitals, mass vaccination clinics, GP surgeries, and community pharmacies.
This will include the creation of vaccination centers that will account for a large part of the vaccines.
The strategy also seeks to draw on the skills of retired physicians and healthcare professionals to help with the vaccination effort.
However, most of the work will fall on healthcare workers, such as GPs, nurses and pharmacists, who will be asked to administer the vaccine program.
Examples of vaccine administration locations will include acute care hospitals such as Tallaght Hospital, St James’s and Beaumont (all in Dublin), as well as Mayo General Hospital, Cavan General Hospital, Cork University Hospital and University Hospital Galway.
The vaccines will also be administered in community nursing units across the country.
Examples of sites for mass vaccination centers are Citywest and the National Exhibition Center in Cloghran.
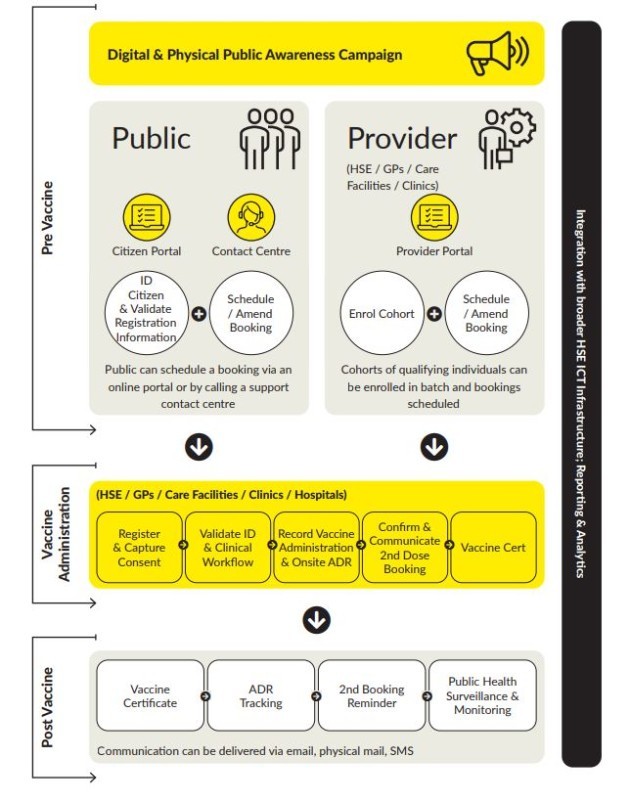
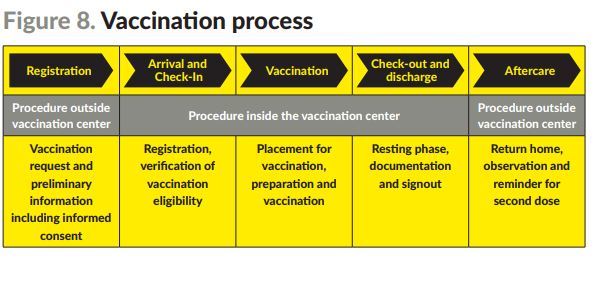
Under what is called the “vaccination route,” the plan describes how members of nominated groups will be invited to register and consent to vaccination.
They will then be offered a scheduled appointment at a designated facility.
The plan says: “The goal is a standard registration process in which all key identifying data, demographic data, any required medical information and informed consent are initiated.”
When a person arrives for their appointment, their previously registered details will be confirmed.
They will then receive their jab and then be discharged after a 15 minute period.
The person will be reminded of the date to return for their second dose and the process will repeat again.
The plan reads: “While the clinical environment will vary, the goal is to provide a consistent process with a common technology and data platform. This will ensure a consistent process, accurate data capture, and timely reporting. “
There will also be a portal on the Health Products Regulatory Authority (HPRA) website to report any suspected side effects experienced after receiving the vaccine.
Each stage of this journey will be enabled and assisted by an ICT system, the plan adds. This ICT system will allow the planning and scheduling of vaccinations, as well as helping to monitor and evaluate the success and effectiveness of the program.
The plan adds: “A functionally rich and proven solution for the proposed vaccination program must be sourced, purchased, implemented and integrated into HSE’s ICT infrastructure by the end of the year.”
HSE CEO Paul Reid said today that the vendor selection process for an IT system has been agreed upon between IBM and Salesforce. He also said that HSE was communicating with the Data Protection Commission about the IT system to be used.
NPHET will also monitor the uptake of the vaccine, while the plan also emphasizes the importance of a clear communication plan to answer any questions the public may have about the safety and efficacy of the vaccine.
It also includes details of when the different vaccines may be available.
January 2021 is the earliest possible date for Moderna and Astrazeneca vaccines to be available. The following month is the first that the Curevac vaccine is available. This is followed by another vaccine in development from Sanofi / GSK in July 2021.
However, Health Minister Stephen Donnelly said today that the launch of the Pfizer / BionTech vaccine could start before the new year if European authorities approve it next week.
In total, Ireland has agreed agreements to access more than 14 million doses of the various vaccines in development.
No news is bad news
Support the magazine
your contributions help us continue to deliver the stories that are important to you
Support us now
The strategy was developed by the High-Level Working Group on Covid-19 Vaccination, which is chaired by former DCU President Professor Brian MacCraith.
Donnelly joined MacCraith, as well as medical director Dr. Tony Holohan, in a briefing this afternoon to outline the strategy.
The cabinet approved the strategy after a meeting this morning.
Dr Holohan, however, added that it is important not to let our guard down now, as Covid-19 will remain with us for the next several months.
HPRA Executive Director Dr. Lorraine Nolan said the European Medicines Agency has been reviewing the data from the Pfizer / BioNTech vaccine on an ongoing basis.
He added that the scientific experts involved in the approval of the vaccine are focused on completing the “detailed and rigorous review” in the shortest time possible.
With reporting by Rónán Duffy, Christina Finn
[ad_2]