[ad_1]
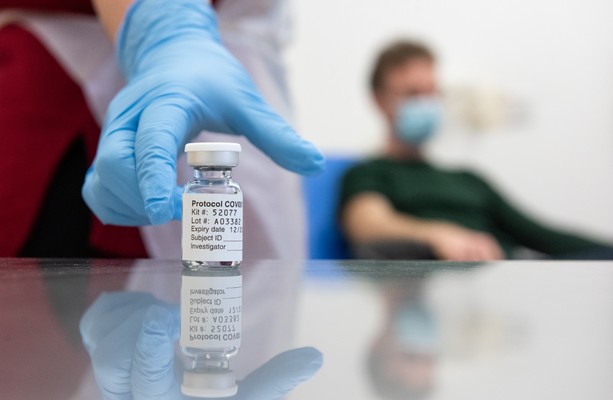
This morning it was announced that the UK formally approved a Covid-19 vaccine developed by Germany’s BioNTech and US giant Pfizer, becoming the first country to do so.
The vaccine has been licensed by the UK Medicines and Health Products Regulatory Authority (MHRA) for emergency use.
The Guardian reports that the UK has purchased 40 million doses of the vaccine, with a company statement saying the first doses will arrive in the UK in the next few days.
“The government today accepted the recommendation of the Medicines and Healthcare Products Regulatory Agency to approve the Pfizer-BioNTech Covid-19 vaccine for use,” the UK health department said in a statement.
“The vaccine will be available across the UK from next week,” the statement said, with priority groups including nursing home residents, health and care staff.
Traditionally, research and development of vaccines can take years, so how has it been approved for use so quickly in the UK?
We’ll see…
How has this happened so quickly?
The timetable for developing and approving a Covid vaccine has been condensed due to the coronavirus crisis.
What is the usual process for developing a vaccine?
Traditionally, vaccine development takes several years and includes several processes, including design and development stages followed by clinical trials, which themselves require approval before starting.
The trials take place in three sequential stages, also known as phases. Research will show whether a vaccine generates antibodies but also protects people from disease. They will also identify any security issues.
Once the trials are complete, the information collected by the researchers is sent to regulators for review.
This is thoroughly analyzed by doctors and scientists before it is approved for widespread use.
Then, after approval from regulators, people can start getting the vaccine.
Is this different due to the pandemic?
The process looks slightly different in trials for a Covid vaccine.
While the initial design and development stages appear similar, the clinical trial phases overlap, rather than taking place sequentially.
But doesn’t that mean that security is compromised?
Although some phases of the clinical trial process have been run in parallel rather than one after the other, the safety checks remain the same as for any new medicine.
The Regulatory Agency for Medicines and Health Products (MHRA) has adopted the phrase “safety is our watchword”.
Regulators have said they will “rigorously evaluate” the data and evidence presented on the safety, quality and efficacy of the vaccine.
And, in most clinical trials, any safety concerns are generally identified within the first two to three months, a period that has already passed for most pioneering vaccines.
How do regulators act so quickly?
Regulators have been conducting “rolling reviews,” meaning that instead of reviewing masses of information at the end of trials, they have been given access to the data while scientists work.
An ongoing review of the vaccine data began several months ago.
This means that regulators can begin to analyze scientific data earlier than they traditionally would, which in turn means that the approval process can be sped up.
Regulators sometimes have thousands of pages of information to go over with a fine tooth comb, which understandably takes time.
Once all available data on the vaccine is submitted, MHRA experts will carefully and scientifically review the safety, quality, and efficacy data – how it protects people from Covid-19 and the level of protection it provides.
Once this is done, the advice of the independent government advisory body, the Commission for Human Medicines (CHM), is sought.
What does “approved for use” mean?
For a drug to be used in the UK, it must be licensed. This means it has gone through all rigorous safety and efficacy checks and the results of clinical trials are trusted by regulators.
By reviewing the data as it becomes available, the MHRA can come to your opinion earlier on whether the drug or vaccine should be licensed without compromising the thoroughness of its review.
So what data will the regulator analyze?
Information provided to the MHRA will include what the vaccine contains, how it works in the body, how well it works and its side effects, and who it is intended to be used for.
This data should include the results of all animal studies and human clinical trials, manufacturing and quality controls, consistency in batch production, and testing of the final product specification.
The factories where the vaccines are manufactured are also inspected before a license can be granted to ensure that the product supplied is of the same consistent high standard.
No news is bad news
Support the magazine
your contributions help us continue to deliver the stories that are important to you
Support us now
What is the difference between the MHRA and the CHM?
The MHRA is the British regulator for medicines and medical devices, which guarantees their safety, quality and efficacy.
The CHM advises ministers on medicines. It is made up of an independent group of advisers responsible for advising on the need and content of risk management plans for new drugs.
It also advises officials on the impact of new safety concerns on the balance of benefits and risks of licensed drugs.
The CHM also offers advice on “applications for both national and European marketing authorizations”.
Haven’t pharmaceutical companies started making vaccines yet?
Yes. Large-scale production and distribution typically begins only after regulatory approval. But in the case of Covid-19 vaccines, pharmaceutical companies have started manufacturing before final approval was granted, taking on the risk of being forced to scrap their work.
Includes reports from the Press Association and © AFP 2020
[ad_2]