For the first time, the innovative method of CRISPR gene modification has been applied to squid, marking a milestone in the scientific study of these creatures – and opening up many new areas of potential research.
CRISPR enables highly secure, fast, and low-cost DNA processing. Simply put, the ingenious molecular action of the method is often described as something that can ‘cut’ and ‘paste’ our genes; in humans, it promises to give us a way to tackle diseases and kill superbugs at a genetic level.
In this case, CRISPR-Cas9 genome editing was used Doryteuthis pealeii (the lungfin-shortened ink) to eliminate a pigmentation gene, exposing the pigmentation normally found in the single eye and within specialized skin cells called chromatophores.
“This is a critical first step toward the ability to exclude genes – and knock them into kephalopods to address a host of biological questions,” said marine biologist Joshua Rosenthal, of the Marine Biological Laboratory (MBL) at the University of Chicago.
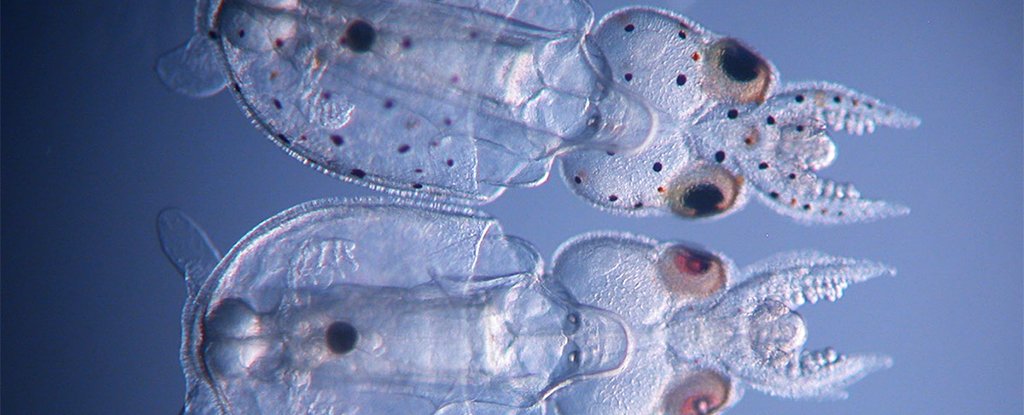
 CRISPR-Cas9 squid. (Karen Crawford)
CRISPR-Cas9 squid. (Karen Crawford)
The lungfin shortened ink is of enormous importance to scientists, and studies of Doryteuthis pealeii go as far back as the fifties have helped make important contributions in the field of neuroscience – including the first description of the nerve impulse.
Cephalopods (squid, octopus, and cuttlefish) have larger brains than any other invertebrate, the ability to alter their own genetic information, and some prefer great party tricks (as it can instantly change color).
Genes in these creatures can edit is an important new development, and one that could see squid join the ranks of model organisms in genetic research, such as fruit flies and zebrafish. The study of evolution, medicine, robotics, materials and even artificial intelligence could all benefit.
“They have developed this great brain and this behavioral disorder completely independently,” Rosenthal told NPR. “This provides an opportunity to compare them with us and see which elements are common, and which elements are unique.”
What makes this new achievement such a success is the delicate way CRISPR had to be applied: overcoming the tough outer layer of the single-celled squid embryo with microscissors and a quartz needle, and then erupting.
 Doryteuthis pealeii in the water. (Roger Hanlon)
Doryteuthis pealeii in the water. (Roger Hanlon)
The timing of the operations was crucial, but after many false starts (and broken needles), the team was able to develop embryos with less pigmented cells and lighter eyes.
It is to be hoped that, because these squid are masters at editing their own genetic code, this research could further our own techniques. The next step is to try the technique in smaller types of inks that are easier to raise (and study) in the lab.
In the future, this method will be massively useful for researchers who test the function of certain single genes, and follow genes that control neural activity in creatures – all options that were simply not available before.
“Now we actually have the opportunity to go in and test what an individual gene does,” marine biologist Carrie Albertin, of the MBL, told NPR.
“This is something that honesty, if you asked me five years ago if we could do that, I would have just giggled and said, ‘I dream about it.’ But, you know, I did not think it would be possible. And yet we are here. “
The study was published in Current biology.
.