Last week, the US Food and Drug Administration (FDA) planned to release an emergency use authorization (EUA) for blood plasma as a treatment against COVID-19. But the authorization is now on hiatus after top experts, namely Dr Anthony Fauci and Dr Francis Collins, confidently said that the evidence was not strong enough to do so at this time, according to a new report.
Dr H. Clifford Lane, deputy clinical director at the National Institute of Allergy and Infectious Disease, said the authorization “is currently pending” while more data is being tested. However, emergency service approval is still possible; Lane notes that it “could still be released in the near future,” according to The New York Times report.
An FDA spokesman on Wednesday did not immediately return Fox News’ request for comment.
MANDATED CORONAVIRUS VACCINATION IN UNIQUE, FAUCI SET
Fauci, the top infectious disease expert, and Collins, director of the National Institutes of Health, reported calling on FDA officials to ‘hold on’ to the authorization, pointing to new data on plasma transfusions of ‘ and the Mayo Clinic. The study found that convalescent plasma with higher antibody levels transferred to hospital COVID-19 patients “significantly reduced mortality” compared to transfused plasma with low antibody levels. However, Fauci and Collins believe that the study strongly lacks data to justify an authorization for emergency use.

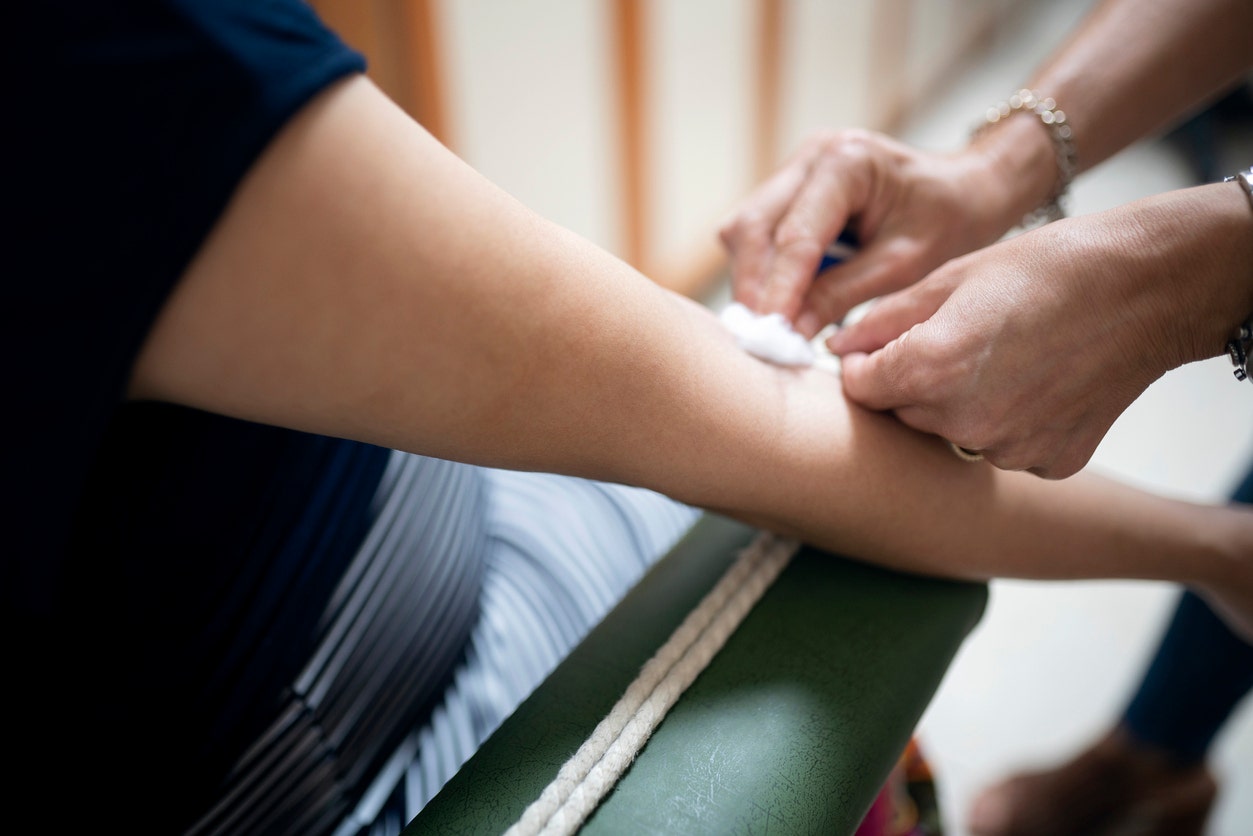
Doctors have accused plasma transfusions of being an effective treatment for coronavirus for months. (iStock)
Doctors have blamed plasma transfusions as an effective treatment for coronavirus for months, and hospitals and the American Red Cross have recruited volunteers to donate plasma to sick coronavirus patients. The idea is that in the plasma antibodies from recovered COVID-19 cases transferred to critically ill COVID-19 patients will help fight or neutralize the disease.
The FDA approves authorizations for emergency use in public health emergencies to expedite rapid medical use (if not approved use of such) to treat or prevent serious diseases where there are no other adequate and available alternatives. For example, on May 1, President Trump announced that the FDA authorized the emergency use of Gilead Science’s experimental antiviral drug remedy for the treatment of coronavirus patients after early results from a clinical study indicated the drug helps speed recovery.
In mid-June, the FDA withdrew the authorization for emergency use of chloroquine and hydroxychloroquine donated to the Strategic National Stockpile to treat certain hospitals with coronavirus patients, as it determined that the two drugs were unlikely to be effective in treating COVID-19 for authorized use in the EUA.
Louis Casiano and James Rogers of Fox News contributed to this report.
CLICK HERE FOR THE FOX NEWS APP