[ad_1]
Title: Phosphine gas in the cloud covers of Venus
Authors: Jane S. Greaves, Anita MS Richards, William Bains, Paul B. Rimmer et al.
Institution of the first author: School of Physics and Astronomy, Cardiff University and Institute of Astronomy, University of Cambridge, Cambridge UK.
State: Accepted in Nature Astronomy, Open Access
“The temperatures and pressures of ~ 40-60 km on the surface of Venus are like Florida, except where the water droplets are highly concentrated sulfuric acid. Venus sucks!
– Hank Green
Observing phosphine
In the summer of 2017, the James Clerk Maxwell Telescope (JCMT) in Hawaii turned to our sister planet Venus. The goal that night was to observe the phosphine molecule or PH3. Why PH3? It is a promising sign of life, if detected on another planet. Phosphine is found in the Earth’s atmosphere and its origin is due to human activity or microbes. PH3 It reacts easily and effectively with oxygen to create phosphorous acid, destroying phosphine. Lifeless on Earth, PH3 Most likely it does not exist in our atmosphere due to how rich in oxygen it is. Hence, an abundance of PH3 in the atmosphere of a rocky planet it could very well suggest life. PH3 has been detected on Jupiter and Saturn, in minute quantities, however, the mechanism that creates PH3 on these planets it occurs in the depths of the planet where there are very high temperatures and pressures, and then it is brought to the surface of these gas giants by convection. Rocky planets have similar environments deep within the planet’s interior, yet the rocky surfaces of Mercury, Venus, Earth, and Mars block the PH3 going up into the atmosphere where it can be observed.
Venus in particular also has a very oxygen-rich atmosphere, so any PH3 that could be created through known chemistry would quickly become H3post3. Based on what this scientific team knew about Venus and the chemistry of phosphine, they were not expecting a phosphine detection. Therefore, when phosphine was detected in 2017, the team decided to do a follow-up observation with the Atacama Large Millimeter / submillimeter Array (ALMA). This telescope would provide higher sensitivity as well as better spatial resolution. They were then able to confirm that another telescope at another time would also detect the presence of phosphine and map the location of the phosphine in the atmosphere of Venus.
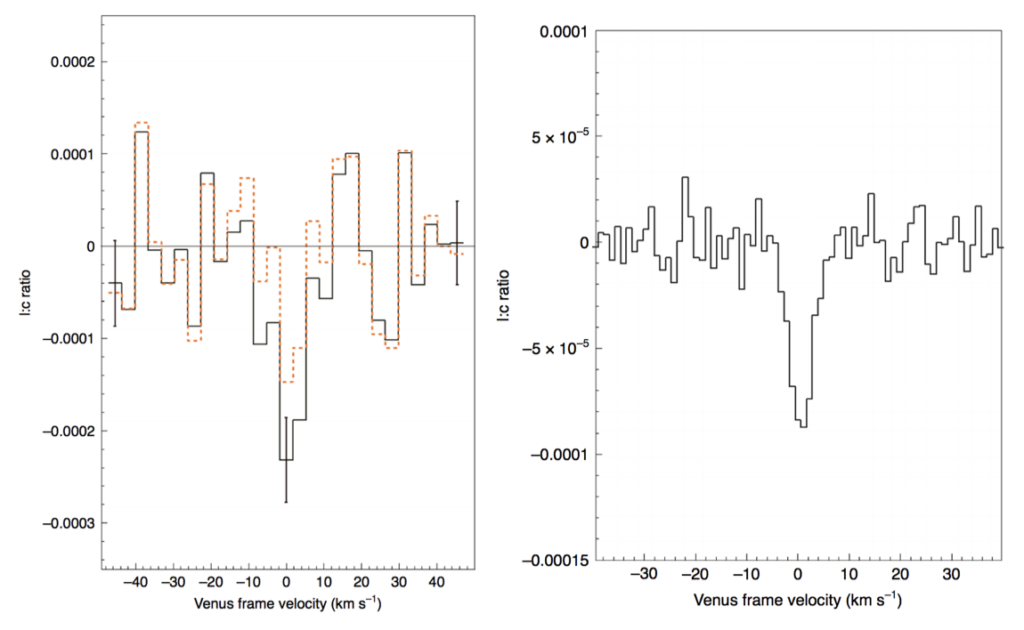
Sure enough, when ALMA looked towards Venus in 2019, phosphine was detected again. The JCMT and ALMA detections matched each other, telling us that not only should it be a real detection, but over the course of two years the amount of PH3 didn’t seem to change. This team went out of their way to make sure it was a real PH3 detection. They ruled out the possibility of another molecule contaminating the observed line and had several people perform data reduction on the observations. This certainly means that PH3 has been detected on Venus! Using the spatial resolution provided by ALMA, they also determined that the PH3 it must exist more than ~ 53-61 km in the atmosphere of Venus, and that the phosphine distribution must be somewhere between completely uniform on the surface and in distinct small patches.
Where he came from?
Using the flux count from the JCMT observation and a model of Venus’s atmosphere, this team determined that the abundance of phosphine is ~ 20 parts per billion or ppb (for every trillion molecules / atoms, 20 of them are PH3). PH shelf life can also be predicted3 in the atmosphere depending on which molecules can destroy the PH3 and the mixture within the atmosphere that raises the PH3 to less habitable environments. This team discovered that the half-life of a PH3 molecule is less than 103 seconds. With the combined knowledge of constant abundance and the half-life of PH3, the PH production rate3 then it can be calculated. They find that PH3 must occur at a rate of ~ 106 – 107 mole cm-two s-one. Now, to explain the observed abundance of phosphine, we need to find some way to produce phosphine at that rate. There has to be some mechanism that produces phosphine, whether in the atmosphere, on the surface, in the subsurface or in space. And this team sought to model every type of mechanism that we know of.
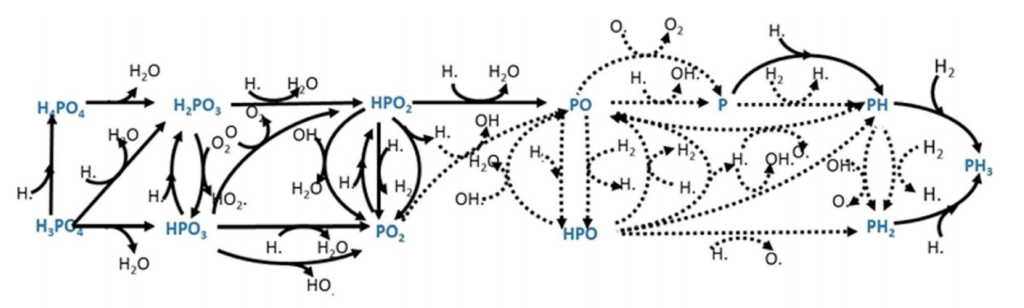
What about known chemical reactions in the atmosphere?
They ran chemical models that used ~ 75 different chemical reactions and thousands of different environmental conditions. None of which even came close to their lower PH limit3 of 10 ppb.
From below the surface of Venus?
They ran simulations of the chemistry that occurs within the subsoil, with the idea that the PH3 could be released to the atmosphere, and they found that the abundance of oxygen within the crust and mantle of Venus was too high to support the PH3 production and launch.
Oooh, here’s an idea. Flash of lightning!
While lightning occurs on Venus and can produce trace amounts of PH3, the team found that the rays did not occur frequently enough and were not effective in producing PH3. In fact, it produced 10,000,000 times less than the amount detected.
How about getting phosphine from meteorites or from a large comet?
Assuming Venus does not receive many more meteorites than Earth does, there is not enough material in the meteorites to produce the detected phosphine levels. And there is no evidence of a major impact on the recent history of Venus.
Volcanoes !!
There are volcanoes on Venus and they can produce phosphine. But to get close to the amount that is observed, Venus would have to be 200 times more volcanically active than Earth, and we see no evidence for that.
Aliens?

Phosphine detection corresponds quite well to Hadley cells inside Venus, where temperatures and pressures are very similar to Earth’s, making them friendly to life. However, what is not very beneficial for life are the incredibly concentrated droplets of sulfuric acid that no life on Earth has been shown to resist. The only ‘life’ that could possibly, perhaps, survive here are small microbes. But life has proven to be adaptive and resilient, so …
Now what?
This is what we know. Astronomers have detected the PH molecule3 into the atmosphere of Venus. This is strange because there are many oxygen-carrying molecules that would easily destroy the PH.3 unless there was some source of constant PH production3 that astronomers cannot identify as of now. This means that there is an unknown chemistry in the atmosphere of Venus. One solution could be that there are Extremophiles that survived Venus’s leakage greenhouse effect billions of years ago, and they exist within a zone of Venus that is slightly habitable. It is quite possible that there are other solutions that involve unknown chemical and environmental conditions within Venus. One thing’s for sure, you’ll never know if you don’t go.
About Jenny Calahan
Hello! I am a third year graduate student at the University of Michigan. I study protoplanetary disks, which set the stage for planet formation. For the past few years, I have been using high resolution ALMA data to extract the 2D thermal structure of different types of discs using thermochemical models. Outside of astronomy, I love to sing tunes, eat Thai food, enjoy handicrafts, and love to travel and explore new places. Visit my website: https://sites.google.com/umich.edu/jcalahan