[ad_1]
Using less than 200 nanograms of einsteinium, half of the world’s supply at the time, scientists have discovered the synthetic element’s binding and spectroscopic behavior for the first time.
Discovered in the rubble after the detonation of the first hydrogen bomb in 1952, einsteinium is a highly radioactive actinide. Since it does not occur naturally on Earth, little is known about its chemistry beyond the fact that it forms some salts of halides and oxides. Doing more than traces means bombarding lighter elements with neutrons over an extended period of time, a process that can only be done in one place in the world, the high flux isotope reactor at Oak Ridge National Laboratory in Tennessee, USA. USA
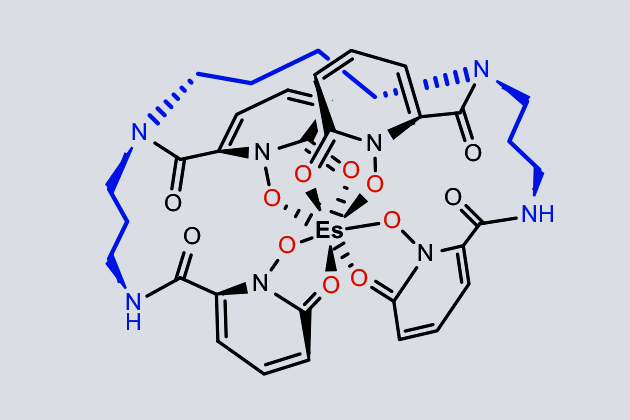
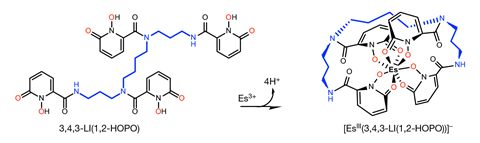
The lab’s latest efforts produced just 400 ng of element 99, half of which went to a team led by Rebecca Abergel from the University of California, Berkeley, Corwin Booth from Lawrence Berkeley National Laboratory, and Stosh Kozimor from Los Alamos National Laboratory. . Despite working with less than 200 ng of the element, the researchers managed to subject einsteinium to X-ray absorption measurements, revealing for the first time its coordination chemistry and spectroscopic behavior.
In some ways, einsteinium behaves similarly to its lighter neighbors on the periodic table, adopting a +3 oxidation state in a complex with an octadentate hydroxypyridinone ligand. However, the short length of the compound’s einsteinium-oxygen bond came as a surprise. Luminescence spectroscopy measurements gave another unexpected result. ‘The way [einsteinium] changes after complexation is in the opposite direction in terms of wavelength and energy change than what happens with the other actinides, ”explains Abergel. The team is now working to confirm why the behavior of einsteinium is so different from other actinides.
Normal rules no longer apply
“ One thing this talks about is that we don’t have good control over what the impacts of relativistic effects are on the chemistry of these elements, ” says Jenifer Shafer, an expert in heavy actinide chemistry at the School of Colorado Mines, USA “The normal rules of quantum mechanics and electronic ordering, things like Hund’s rule, seem to dissolve as you enter this part of the periodic table.”
One of the most exciting things about doing actinide chemistry is that there are elements that we can write the textbook for.
Stosh Kozimor, Los Alamos National Laboratory
Shafer is impressed with the Berkeley team’s ability to make the experiments happen in the first place. “I work with einsteinium a bit, but I can’t imagine the challenge of trying to coordinate getting a few hundred nanograms and getting meaningful chemical data,” he says.
You can’t really see [the material when it arrives]”Recalls UC Berkeley researcher Katherine Shield, who did much of the bench chemistry. “It comes in a small bottle, but the only way to know that you are really working with it is by using radiation detectors.” Aside from the miniscule amount the researchers had to work with, the 275-day half-life of einsteinium meant they were losing material over time. “You really, really hope you don’t accidentally drop a drop while doing the chemistry,” Shield says.
Even before receiving the einsteinium, the team had carefully laid out the experiments they wanted to perform, and in what order, to minimize material loss. “We planned a lot in advance and nothing went according to that plan,” laughs Abergel. Finding that their sample contained large amounts of californium, they had to abandon planned X-ray diffraction experiments and turn to X-ray absorption spectroscopy, allowing them to ignore the contaminant.
After preparing the einsteinium complex at Berkeley, team member Korey Carter drove the precious and dangerous sample for an hour to Stanford’s synchrotron radiation light source. Once there, handling half the world’s supply of einsteinium was “absolutely terrifying,” recalls Kozimor. “You need a well-rehearsed team and strong nerves.”
“It’s also a real privilege to do this kind of science,” says Abergel. Understanding the chemical behavior of einsteinium could help scientists produce and purify it in order to produce even heavier elements. “Einsteinium targets would make the potential identification of the island of stability more plausible, which is a big problem for nuclear physicists,” Shield explains. The island of stability is a predicted set of superheavy isotopes that can have considerably longer half-lives than their close neighbors.
For now, the team will continue to work with the remaining einsteinium sample, conducting electron microscopy, radioanalysis and separation experiments. ‘One of the most exciting things about doing actinide chemistry is that there are elements that we can write the textbook for. It’s truly exploratory, ”says Kozimor.