
[ad_1]
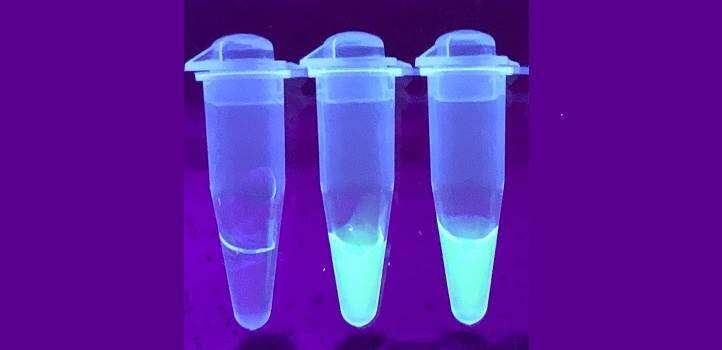
One method of visualizing the result is to illuminate the sample with ultraviolet light and a detector analyzes the light to report the amount of viral RNA. Credit: Ali et al.
A simple COVID-19 test kit combines virus amplification with a CRISPR-Cas system for effective detection of SARS-CoV-2. The kit, called iSCAN, uses reagents that can be manufactured locally.
“Our entire iSCAN procedure can be completed in less than an hour and can be easily adopted as a point-of-care detection system at airports and borders,” says KAUST Ph.D. student Ahmed Mahas.
The current gold standard in SARS-CoV-2 testing is the PCR test, in which DNA primers recognize specific RNA sequences in the viral genome that are then copied using a specific enzyme. This “amplification” process facilitates the detection of the originally small amounts of viral RNA present in nasopharyngeal swabs taken from patients. This test can reliably detect whether a person actually has the virus without providing too many false positive or negative results. But you need highly qualified personnel to perform the test, which is performed in multiple steps in central laboratories with sophisticated equipment.
iSCAN, developed by a team led by KAUST bioengineer Magdy Mahfouz, overcomes many of the disadvantages of the PCR test while providing relatively reliable results.
Significantly, the test reagents were manufactured at KAUST. This includes the enzymes required for amplification and another enzyme that specifically detects the viral sequences within the copied material. The availability of reagents and equipment has been a major obstacle since the start of the COVID-19 pandemic.
To use iSCAN, the contents of a patient’s sample, collected with a nasopharyngeal swab, are placed in a small test tube that contains the DNA primers and enzymes that can amplify the SARS-CoV-2 genetic material. The contents were incubated at a temperature of 62 degrees Celsius for half an hour. This process is known as RT-LAMP. Once enough viral RNA is amplified, a drop containing the Cas12 enzyme is added to the mixture and left for another 15 minutes. This enzyme only recognizes the viral RNA that belongs to SARS-CoV-2, overcoming a problem with RT-LAMP, where false amplification and cross contamination can be a problem.
Finally, one of two methods can be used to visualize the result. One is to shine the sample with ultraviolet light, with a detector that analyzes the light that comes out of it to report the amount of viral RNA. The other approach is to insert specially designed strips into the tubes, similar to those used in pregnancy tests. Both approaches work well, although the UV light method provided more accurate results.
The scientists tested their kit on synthesized viral RNA and real patient samples. “We are now improving and simplifying our system for users to bring our iSCAN detection kit to market,” says KAUST research scientist Zahir Ali.
Follow the latest news on the coronavirus (COVID-19) outbreak
Zahir Ali et al. iSCAN: a CRISPR-Cas12 module coupled to RT-LAMP for rapid and sensitive detection of SARS-CoV-2, Virus research (2020). DOI: 10.1016 / j.virusres.2020.198129
Provided by King Abdullah University of Science and Technology
Citation: For rapid COVID-19 tests, iSCAN can be adopted for airports and public transportation (2020, November 5) Retrieved November 5, 2020 from https://medicalxpress.com/news/2020-11-quick-covid -iscan-airports. html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.
[ad_2]