[ad_1]
Five Chinese COVID-19 vaccine candidates are in phase III clinical trials in countries such as the United Arab Emirates, Brazil, Pakistan and Peru, according to Foreign Ministry spokesman Zhao Lijian on Wednesday.
He presented that the five Chinese vaccines are: two inactivated vaccines developed by the China National Pharmaceutical Group (Sinopharm), an inactivated vaccine developed by Sinovac Biotech Co., an adenoviral vector vaccine developed jointly by the Academy of Military Sciences and the company of Chinese biotechnology CanSino and a recombinant protein vaccine developed by Anhui Zhifei Longcom Biologic Pharmacy.
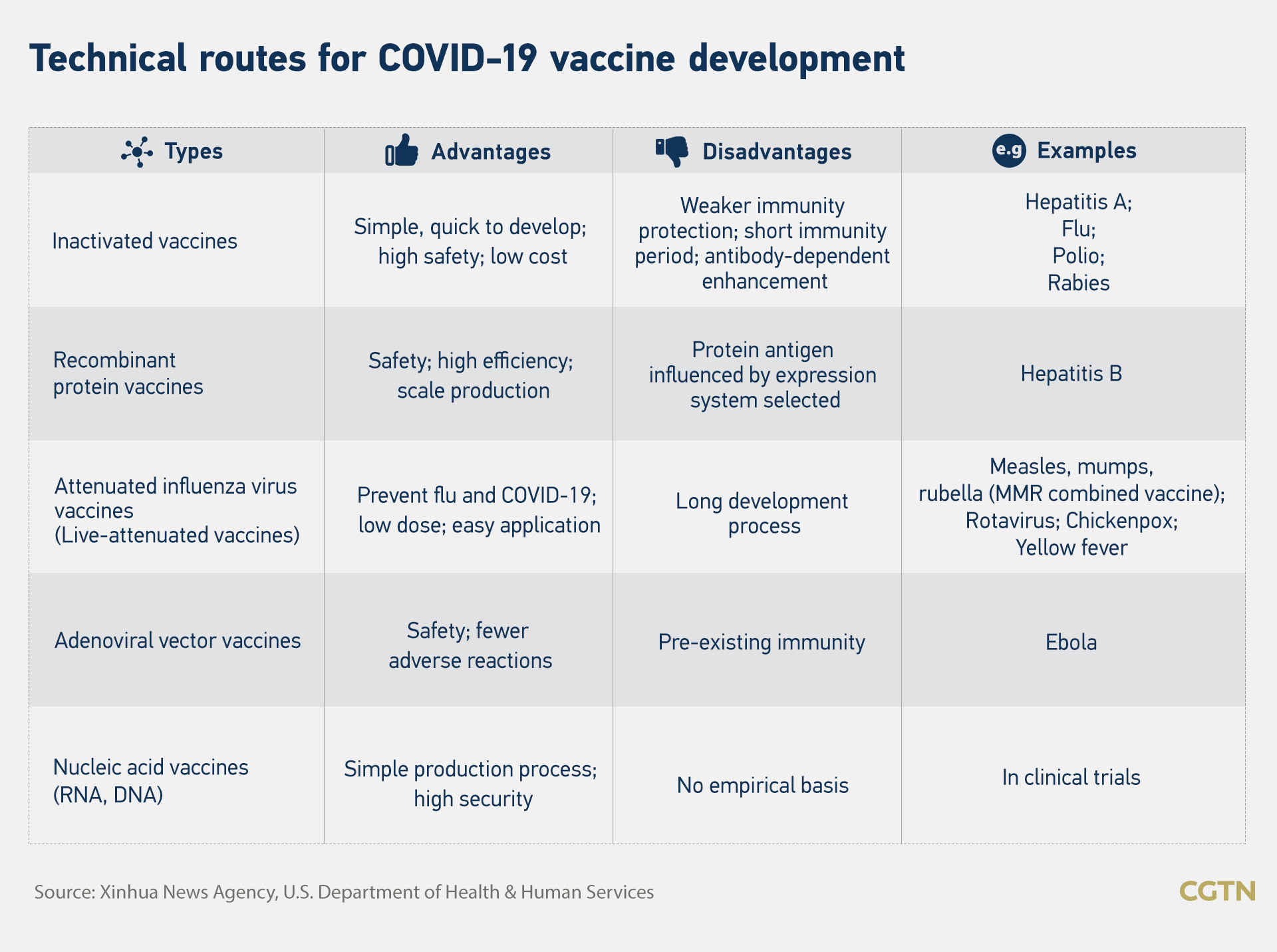
“After the pandemic broke out, the Chinese government established as soon as possible five technical routes that include inactivated vaccine, recombinant protein vaccine, adenoviral vector vaccine, attenuated influenza vaccine and nucleic acid vaccine,” Zhao said.
read more: Tracking COVID-19 Vaccines
He added that, in addition to cooperating with the United Arab Emirates, Brazil, Pakistan and Peru, China also maintains close cooperation with international organizations such as WHO, Gavi, Vaccine Alliance and Coalition for Epidemic Preparedness Innovations.
China also joined the WHO-sponsored Accelerator Access to COVID-19 Tools (ACT) initiative and the Solidarity Trial for COVID-19 and COVAX treatments to promote the fair distribution of vaccines.
“We hope that Chinese vaccines will be included in the COVAX procurement list as soon as possible after their development, which will contribute to the accessibility and affordability of vaccines in developing countries,” Zhao said.