[ad_1]
It has been 17 years since the SARS-CoV coronavirus threatened to erupt into a global pandemic. Thanks to rapid efforts to contain the outbreaks of the infection, the world’s population was spared the worst.
This time we weren’t so lucky. What makes SARS-CoV-2 so much more infectious than its predecessor is a question we’re now a little closer to solving, with researchers discovering another way the virus enters our cells.
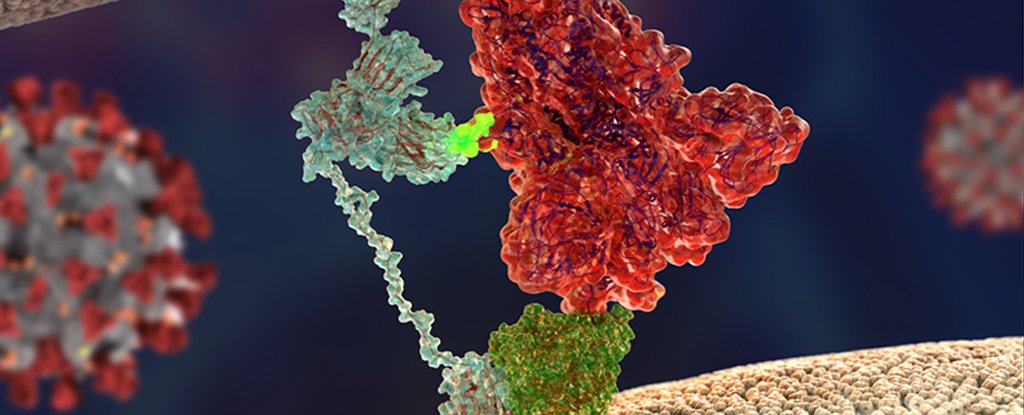
Researchers from the Technical University of Munich in Germany and the University of Helsinki in Finland led a study that found that a receptor called neuropilin-1 gives the new coronavirus an advantage in infecting our tissues.
This particular protein is relatively abundant in the cells that line the nasal cavity, making it very easy for the virus to establish a home within our bodies, generate a family of viruses, and then spread to a new host.
Earlier this year, a receptor called angiotensin-converting enzyme 2 (ACE2) was found to help coronavirus bind to cell surfaces, while an enzyme called transmembrane type II serine protease (TMPRSS2) is crucial to achieving entry.
This type of molecular selection does a good job of explaining why both SARS coronaviruses wreak havoc on a variety of tissues in our body, from the lining of our lungs to our digestive tract.
But it doesn’t say why one virus spreads better than the other.
“The starting point of our study was the question of why SARS-CoV, a coronavirus that caused a much smaller outbreak in 2003, and SARS-CoV-2, spread in such a different way even if they use the same main ACE2 receiver “. says virologist from the University of Helsinki Ravi Ojha.
A crucial piece of the puzzle appeared when comparing the two viral genomes; SARS-CoV-2 had collected sequences responsible for producing a series of spiny “hooks” not unlike those used by other nasty pathogens to latch onto host tissues.
Compared to its older relative, the new coronavirus had acquired an ‘extra piece’ in its surface proteins, which is also found in the spikes of many devastating human viruses, including Ebola, HIV, and highly pathogenic strains of avian influenza. among others. “says Olli Vapalahti, also a virologist at the University of Helsinki.
“We thought this might lead us to the answer. But how?”
In consulting with colleagues around the world, the researchers focused on neuropilin-1 as a common factor.
Normally, this receptor plays a role in the response to important growth factors in tissue development, especially between nerves. But for many viruses, it is a convenient handle to hold onto host cells long enough to enter.
Electron microscopy of the surface peaks coating the SARS-CoV-2 particles certainly hinted at the possibility of a receptor relationship.
To help confirm this, the researchers made use of specifically selected monoclonal antibodies to block access to the garden variety neuropilin-1, but not to the mutant varieties modified to have a slightly different structure.
Sure enough, ‘pseudoviruses’ with SARS-CoV-2 proteins (great for watching viruses get into cells without worrying about all the complicated replication business that follows) had a much harder time entering when neuropilin-1 was blocked.
“If you think of ACE2 as a door lock to enter the cell, then neuropilin-1 could be a factor that directs the virus to the door,” says Balistreri.
“ACE2 is expressed at very low levels in most cells. Therefore, it is not easy for the virus to find doors to enter. Other factors such as neuropilin-1 could help the virus to find its door.”
With neuropilin-1 expressed in large quantities in nerve tissues within the nasal cavity, we could imagine that SARS-CoV-2 has a convenient red carpet unfolded the moment we sniff out an infected drop.
A close look at tissue samples expressing neuropilin-1 taken from deceased COVID-19 patients raised suspicions, while an experiment with mice helped confirm the role of the receptor in helping the virus enter our nervous system.
Whether this could help explain why SARS-CoV-2 infections can have such a traumatic impact on brain function is a question for future research.
“We could determine that neuropilin-1, at least under the conditions of our experiments, promotes transport to the brain, but we cannot draw any conclusion as to whether this is also true for SARS-CoV-2. It is very likely that this pathway is suppressed by the immune system in most patients, “says neurologist Mika Simons of the Technical University of Munich.
It’s tempting to imagine new forms of antiviral medication on the horizon. Although as fast as SARS-CoV-2 reveals its criminal talents, simply blocking cell receptors is likely bad news for our health.
That is not to say that discovery is not without opportunities.
“Currently, our laboratory is testing the effect of new molecules that we have specifically designed to interrupt the connection between the virus and neuropilin,” says Balistreri.
“Preliminary results are very promising and we look forward to obtaining validations Live in the near future.”
This research was published in Sciences.