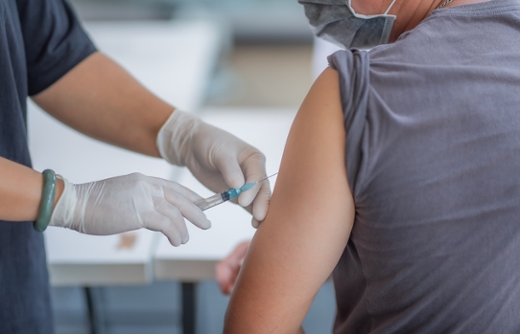
An investigational vaccine designed to protect against coronavirus disease 2019 (COVID-19) was generally well tolerated and resulted in the production of both binding and neutralizing antibodies in healthy adult volunteers, according to interim results from a phase 1 trial published today. at New England Journal of Medicine.
The experimental vaccine is being jointly developed by researchers at NIAID and Moderna, Inc. of Cambridge, Massachusetts. Manufactured by Moderna, mRNA-1273 is designed to induce neutralizing antibodies to a portion of the coronavirus “spike” protein, which the virus uses to bind to and enter human cells.
The Emory University Vaccine Evaluation and Treatment Unit (VTEU) was one of two sites in the nation where the COVID-19 vaccine was tested.
“These interim results are very encouraging,” says Evan Anderson, MD, the trial’s principal investigator at Emory. Anderson is an associate professor of medicine and pediatrics at Emory University School of Medicine and Children’s Healthcare in Atlanta.
“While there is still a lot of work to be done before we have a vaccine that is safe and effective against COVID-19, this study provides critical information about the vaccine’s safety. Importantly, the vaccine resulted in a robust immune response. “
The Emory-based Infectious Disease Research Consortium named the leader of the trial as Lisa A. Jackson, MD, MPH, of the Kaiser Permanente Washington Health Research Institute in Seattle, where the first volunteer received the vaccine on March 16. The published interim report details the initial results of the first 45 volunteers between the ages of 18 and 55. Emory enrolled 17 of those volunteers at Hope Clinic or Emory Children’s Clinic.
The interim analysis includes test results that measure the levels of neutralizing activity induced by the vaccine up to day 43 after the second injection. Two doses of the vaccine elicited high levels of neutralizing antibody activity that were above the average values observed in convalescent sera obtained from people with COVID-19 confirmed disease.
There were no serious events reported. More than half of the volunteers reported fatigue, headache, chills, myalgia, and injection site pain, and systemic adverse events were more common after the second vaccination and in those who received the highest dose of vaccine. .
“We are proud to be involved in finding a vaccine to protect against COVID-19 by enrolling participants in this critical study,” says Nadine Rouphael, lead investigator contact at Emory’s VTEU. Rouphael is also interim director of the Hope Clinic at the Emory Vaccine Center and associate professor of medicine (infectious diseases) at Emory University School of Medicine.
“The increasing rates of COVID-19 infection across the country show that the need for a vaccine has become more urgent.”
Anderson and Rouphael are co-authors of the study published in NEJM.
The Emory and Seattle VTEUs are part of the NIAID Infectious Diseases Clinical Research Consortium, which supports the trial. The consortium is led by co-principal investigators David S. Stephens of Emory University and Kathleen Neuzil of the University of Maryland School of Medicine. Dr. Stephens is a professor and chair of the Department of Medicine at Emory University School of Medicine and vice president of research at Emory’s Woodruff Health Science Center.
“As the largest clinical trial network in the NIH Division of Microbiology and Infectious Diseases, we are working to accelerate research that will identify a vaccine to combat COVID-19,” says Stephens. “We are excited about the promise of these early results and ready for the challenging work that awaits us.”
A phase 2 clinical trial of mRNA-1273, sponsored by Moderna, Inc., began enrollment in late May and there are plans to launch a phase 3 efficacy trial in July 2020..
Additional information on trial design is available at clinictrials.gov using identifier NCT04283461. This trial was supported in part by NIAID grants UM1AI148373 (Kaiser Washington), UM1AI148576 (Emory University), and UM1AI148684 (Infectious Diseases Clinical Research Consortium). The Coalition for Innovation in Epidemic Preparedness (CEPI) provided funding for the manufacture of the Phase 1 material of mRNA-1273.
Learn more about Research COVID-19 from Emory University
About the Infectious Diseases Clinical Research Consortium
Composed of the Vaccine Evaluation and Treatment Units (VTEU) and the IDCRC Leadership Group, it was formed in 2019 to support the planning and implementation of infectious disease clinical research that efficiently addresses NIAID’s scientific priorities. The consortium includes infectious disease leaders and clinical researchers from Emory University, the University of Maryland School of Medicine, Baylor College of Medicine, Cincinnati Children’s Medical Center and the University of Cincinnati, FHI360, the Center for Research of the Cancer Fred Hutchinson, Johns Hopkins University, Kaiser Health Care, New York University, Saint Louis University, Vanderbilt University Medical Center, University of Alabama at Birmingham, University of Rochester, University of Washington, and NIAID. For more information on IDCRC, visit www.IDCRC.org.
.