“It has been delayed,” said Dr. Carlos del Rio, an infectious disease expert at Emory University School of Medicine and one of the investigators for the Modern vaccine.
In mid-June, one of the vaccine testing centers, the University of Illinois at Chicago, said it expected the test to begin on July 9.
“We probably won’t start until July 27,” del Río said. “But July 27 would still be absolutely surprising. Even if it happens in early August, that is still surprising. This is going at a speed that no other vaccine has used.”
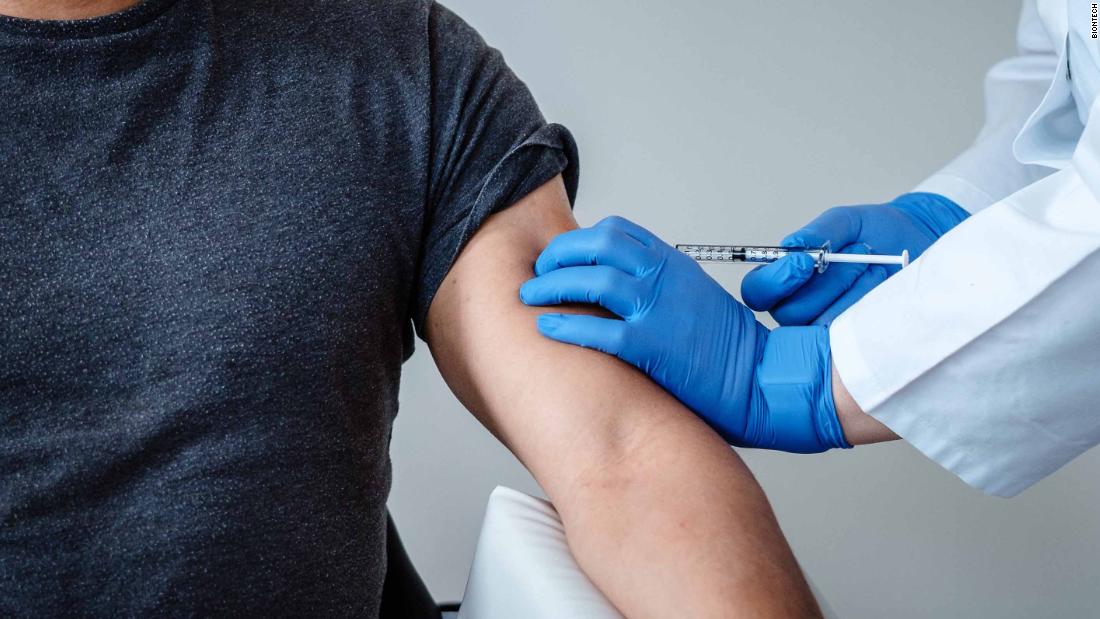
Pfizer Vaccine Sees “Encouraging” Results
“These clinical findings for the BNT162b1 RNA-based vaccine candidate are encouraging and strongly support accelerated clinical development and manufacturing at risk to maximize the opportunity for rapid production of a SARS-CoV-2 vaccine to prevent COVID disease- 19 “. The researchers wrote in the preprinted document, sponsored by BioNTech and designed by Pfizer.
For the initial study, 45 participants between the ages of 18 and 55 were randomly assigned to receive a certain dose of the vaccine or placebo. Twelve participants received two 10-microgram doses 21 days apart; 12 received two doses of 30 micrograms 21 days apart; 12 received a single 100 microgram dose on the first day; and nine received placebo, according to the study.
Within seven days of the vaccine injection, some participants who received one dose reported pain at the injection site, fever, or sleep disorders, but “no serious adverse events were reported,” according to the document.
The researchers found that the vaccine generated antibodies to the coronavirus in all participants 28 days after receiving a single injection of 100 micrograms or seven days after receiving a second dose of 10 or 30 micrograms.
Pfizer and BioNTech announced Wednesday that these preliminary data will help them determine a dose level for the vaccine and then select which of their multiple vaccine candidates will advance to a larger Phase 2/3 global study, possibly starting as soon. like this month.
However, this Pfizer vaccine is not the only one showing positive early data.
Vaccine developer: ‘We are very excited’
The company said 94% of people in the Phase 1 trial demonstrated general immune responses at six weeks after receiving two doses of the INO-4800 vaccine and at eight weeks, the vaccine regimen was found to be safe. and well tolerated without serious reactions
There were 40 healthy adult volunteers, ages 18 to 50, in those preliminary tests, the company noted. Since then, the Phase 1 trial has expanded to include older adults in additional cohorts, and there are plans to launch a Phase 2/3 trial this summer.
“We are greatly encouraged by the positive interim safety and preliminary results of the cellular and humoral immune response to date, as well as by the inclusion of INO-4800 in Operation Warp Speed,” said Dr. J. Joseph Kim, president and CEO of Inovio. The company announcement.
“We hope to urgently advance INO-4800, as it is the only nucleic acid-based vaccine that is stable at room temperature for more than a year and does not require freezing in transport or years of storage, which are important factors in implementing mass immunizations to combat the current pandemic, “Kim said in part.
The INO-4800 phase 1 clinical trial enrolled volunteers at two US locations who were assigned to a 1.0 mg or 2.0 mg dose group, and in those groups, each participant received two doses of INO-4800 with four weeks apart, the company said.
Some participants said they experienced redness where the vaccine was injected into the skin, but “no serious adverse events were reported,” Inovio’s announcement said.
FDA Commissioner: US ‘On Target’ to Receive Vaccine Later Year or Early Next
The United States remains on target to have a Covid-19 vaccine available by the end of the year or early next year, US Food and Drug Administration Commissioner Dr. Stephen Hahn said Thursday.
“We expect two of these vaccines to reach the final stage of clinical trials, which are large clinical trials, this month,” said Hahn. “We are on target to reach a vaccine by the end of the year or early next year, so I am cautiously optimistic. Of course, it depends on the data generated from the trial.”
When Wisconsin Senator Tammy Baldwin asked for details on the vaccine’s prospects, Gary Disbrow, acting director of the U.S. Advanced Biomedical Research and Development Authority, said he was not yet able to release that information, but he hopes to announce the complete portfolio soon.
“I cannot specifically mention some of the companies. We are in active negotiations with many of them. One I can mention is AstraZeneca, where we already have a very broad contract covering both research, development and acquisitions,” said Disbrow Baldwin.
“I think your specific question may be the composition of the portfolio under Operation Warp Speed, which I can’t talk about today,” Disbrow said Thursday. “But we are moving and negotiating contracts very quickly and, hopefully, in the very near future, we will be able to make a full portfolio announcement under Operation Warp Speed.”
Baldwin then requested only a number of how many contracts have been finalized vs. how many are still in negotiations.
“So I already told you what AstraZeneca is about and we have many other candidates that we are working with,” Disbrow replied.
Baldwin asked again how many in all.
“More than one,” Disbrow said, and laughed.
Pharmaceutical company AstraZeneca announced in May that it received more than $ 1 billion from BARDA to support the development, production, and delivery of a coronavirus vaccine. AstraZeneca has been working with the University of Oxford in the UK on the development of the vaccine, with the aim of starting the first deliveries in the fall.
Overall, Operation Warp Speed aims to deliver 300 million doses of a safe and effective vaccine for Covid-19 in January in the United States.
.