
[ad_1]
For stocks, look at Golden Unicorn’s authoritative, professional, timely, and comprehensive analyst research report and it will help you seize potential thematic opportunities!
UK epidemic prevention measures updated! WHO: China’s vaccine is effective, A shares immediately skyrocketed by 100 billion
Source: China Fund News
Amman
The second overseas epidemic struck, and medical stocks once again became the prettiest “guys” on the A-share market.
At the close of noon, the concept of medical devices continued to rise. At the close of this edition,Bohui Innovation、Zhengchuan Stock、Kangtai MedicineDaily limit. The block closed 2.6% higher overall.

In addition, the WHO chief scientist confirmed that China’s new corona vaccine is effective, and the stocks of the new corona vaccine concept have once again received the funding search. At the closure,Saisheng PharmaceuticalDaily limit,Cansino-u went up almost 15%,ZhifeiwithKangtai BioIt went up more than 10%.

The market value of the entire sector soared 98.4 billion yuan from the previous trading day, and increased by about 120 billion yuan in two days.
New diagnostics bounced to peak of first wave (Golden Unicorn analyst)
Britain announced: lockdown for six months
In the weak market, such a strong performance of medical device stocks is no stranger to the second outbreak of epidemics abroad.
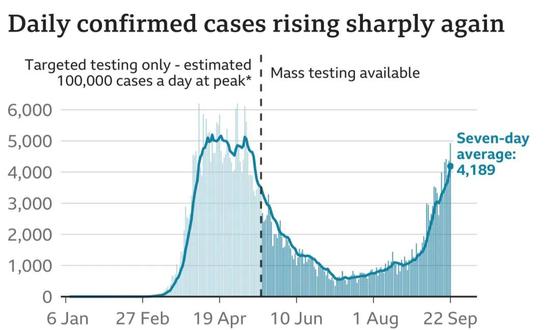
According to BBC statistics, the number of diagnoses in the UK has increased considerably recently. In the week ending September 22, the average number of new diagnoses per day was 4,189, which is very close to the peak of the first wave.
In response to the second outbreak of the new crown, British Prime Minister Boris delivered a national address at 8 p.m. local time on September 22, announcing six new regulations for the prevention and blocking of epidemics, and said that the new regulations will have a duration of 6 months.
The new regulations established that the United Kingdom will launch the following six new regulations on preventing and blocking epidemics from this Thursday (24 September):
1. People should work at home as much as possible.
2. As of Thursday (September 24), all bars and restaurants in England must close after 10pm. Customers are also prohibited from ordering in front of the bar counter, and only waiters can place orders for customers at the table.
3. Employees, waiters and taxi drivers in bars and shops must wear masks, and customers must also wear masks when not seated.
4. People who do not wear masks as required will be fined £ 200.
5. The upper limit on the number of people attending the wedding has been lowered from the current 30 to 15 people, and the upper limit on the number of mourners at funerals is 30.
6. It will not be possible to open all types of venues on time as of October 1, and indoor team sports, including futsal, will be prohibited.

Many European countries restart prevention and control measures
European stock markets rebound
In the first wave of the epidemic, due to inadequate prevention of the epidemic in many European countries, countries such as Italy and Spain became the “epicenter” of the epidemic.
In light of the intensified rebound in the second wave of the epidemic, many European countries have reinitiated prevention and control measures. Unlike the nationwide “city closures” generally adopted in the spring, the relevant restrictive measures currently target areas with severe epidemics.
In Spain, the government of the Community of Madrid has decided to implement a traffic restriction policy for at least 14 days in 37 basic health districts in various urban areas and surrounding municipalities of Madrid as of the 21st to restrict the movement of people; the number of public or private concentrations in the autonomous area. The upper limit has also been adjusted to 6 people.
In France, the Auvergne-Rhône-Alpes region took the lead in tightening measures to prevent epidemics. Since the 22nd, the sale and consumption of alcoholic beverages between 8:00 pm and 6:00 am was prohibited; Marseille and Bordeaux announced restrictions o It is forbidden to gather, partially or completely close non-essential places open to the public and apply improved measures such as travel restrictions; the Alpes-Maritimes province prohibits gatherings of more than 10 people in parks, gardens and beaches in Nice.
In Austria, the government began implementing stricter epidemic prevention measures from 21: reducing the upper limit for private indoor activities from 50 to 10 people; tightening the restaurant industry epidemic prevention regulations, and each table cannot exceed 10 guests.The business must close at 1 o’clock; the scope of the mandatory wearing of masks is expanded, requiring that the masks be worn in markets and outdoor displays, and the masks must be worn in all dining venues except for sitting and eating.
In Ireland, the capital Dublin has implemented a three-tiered epidemic response mechanism since 19, prohibiting non-essential personnel from entering and leaving the area and delaying the resumption of bars.
In some areas with severe epidemics in Greece, the suspension of local schools was extended until September 25.
On September 21, affected by the panic of the rebound of the new corona epidemic, the main stock exchanges of the world fell and the three main European exchanges fell simultaneously. Concerns about the possibility of London “shutting down” again triggered a sell-off, with the London stock market losing £ 51.7 billion in value that day.
Following the WHO’s affirmation of Italy’s prevention and control measures and the official implementation of the blockade prevention and control measures, on September 22, European stock markets generally recovered.

WHO: China’s vaccine has proven effective
According to the Xinhua News Agency, the chief scientist of the World Health Organization Sumia Swaminatan said that the new research and development project of the new Chinese crown vaccine is very active and that some vaccines have proven their effectiveness in the current clinical trials.
Sumia Swaminathan noted that China has several candidate vaccines and the results of phase I and phase II clinical trials are promising.



Sumia Swaminathan also said that before the end of this year, there may be one or two successful vaccine candidates. It is expected that by 2021 2 billion doses of vaccine can be produced. Fortunately, the mutation of the new coronavirus appears to be much smaller than that of the influenza virus and key parts, such as the severity of the disease and the immune response, have not shown a mutation.
At present, China is not only the largest exporter of anti-epidemic materials, but also a country with a high degree of activity in the development of new corona vaccines.
As of September 8, a total of 9 vaccines worldwide have entered phase III clinical trials, of which 4 are from China. They were developed by the team of Academician Chen Wei from the Academy of Military Medicine of the Academy of Military Sciences in cooperation with Kangsino Biology, Kexing Biological, Sinopharm Wuhan Institute of Biological Products and China Biological Beijing Institute of Biological Products.
Although nine vaccines worldwide have entered phase III clinical trials, the testing process has not been straightforward.
On September 8, the vaccine developed by the University of Oxford and AstraZeneca Pharmaceuticals Co., Ltd., which has entered phase III clinical trials, reported that the subjects had “suspected serious adverse reactions.” AstraZeneca Pharmaceuticals immediately issued a statement, voluntarily stopping vaccination in all clinical trials.
On September 14, Oxford University announced that the Brazilian regulatory agency approved the resumption of the vaccine developed by AstraZeneca for subjects in clinical trials in Brazil.
On September 15, Pfizer in the United States also reported that the new corona vaccine it developed had mild to moderate side effects in phase III clinical trials.
In China, in the international phase III clinical trial of the new recombinant corona vaccine (Ad5-nCoV) developed jointly by the team of academician Chen Wei and Cansino, all subjects in the first group were enrolled and vaccinated. Kexing Biosciences also recently launched Phase III clinical trials in Turkey.
At present, there are no reports of serious adverse reactions or archival trials of the four vaccines in China.
Affected by this news, the biological vaccines index also opened higher on the 23rd. At the close, the index rose 3.77%.

The market value of the entire sector soared 98.4 billion from the previous trading day.

Sina Statement: Sina.com publishes this article for the purpose of conveying more information, and does not mean that I agree with their views or confirm their description. The content of the article is for reference only and does not constitute investment advice. Investors trade accordingly at their own risk.
Disclaimer: The content provided by Wemedia is derived from Wemedia and the copyright belongs to the original author. To reprint, contact the original author and obtain permission. Opinions in the article represent the author only, not Sina’s position. If the content involves investment advice, it is for reference only and should not be used as an investment basis. Investing is risky, so be careful when entering the market.

Massive information, accurate interpretation, all in the Sina Finance APP
Editor in charge: Chen Zhijie