[ad_1]
Investment Research Report
[Informe de investigación incondicional]The product can treat the new crown, but 120 million kidney disease patients still need it! This king of medical devices has occupied 80% of the national market and the product has been included in 6 national health insurance!
[ETF Investment Daily]Banks have risen, which ETF to choose? SSE 50ETF suffered 4 consecutive days of decline
[Informe de investigación incondicional]Materials with “super powers”! As a cornerstone of the aerospace industry, 40% of supply is dependent on imports and production capacity remains insufficient. These pioneers are about to take off (list)
[Orden de montaje del administrador de fondos]Harvest Star Fund Managers Collectively Enter Weibo
Original Caption: Kexing’s New Corona Vaccine Approved for Conditional Inclusion, Demand for Syringes Skyrocketed! Prices Rise More Than 2x, Concept Stocks Boiled
Source: China Securities Journal
Good news, the new Kexing Zhongwei coronavirus vaccine has been approved for conditional inclusion!
As new national corona vaccines have been approved for conditional marketing, the overlapping global vaccination plan is advancing rapidly and, for a time, syringes have become rigid demand.
According to media reports,International logisticsThe white paper recently published by the giant DHL and McKinsey shows that after the advent of the new corona pneumonia vaccine, worldwide orders will exceed 10 billion doses.priceCompared to before the epidemic, it has more than doubled.
Related national listingthe companyExecutivesHe told Zhongzheng Jun that the price of the company’s syringes has increased by 50% in the past, and order calls have been affected. The company plans to expand production and is expected to double its production capacity in May. There are also publicly traded companies claiming that the price of the company’s syringes has doubled in the past.In the face of increasing demand for syringes, listed companies are busy expanding production and plan to continue through the Spring Festival .OvertimeAdditional points.
Fueled by the good news, on February 5, stocks related to syringes rose sharply. among them,Wanbond、CondeletDaily limit,Sanxin MedicalMany stocks were up more than 7%.
New coronavirus vaccine Kexing Zhongwei
Conditional listing
On February 6, Kexing Holdings BiotechnologyLimited liability companyIt was announced that the State Food and Drug Administration had approved the conditional list of the new type of coronavirus vaccine developed by the company’s subsidiary Kexing Zhongwei on February 5 in China.
Kerrlive is made by inoculating African green monkey kidney cells (Vero cells for short) with the new coronavirus (strain CZ02), which is obtained by cultivating, collecting virus fluid, inactivating the virus, concentrating, purifying, and adsorbing on aluminum hydroxide . . The vaccine is suitable for the vaccination of people over 18 years of age to prevent disease (COVID-19) caused by the new coronavirus infection (SARS-CoV-2). The basic immunization schedule for the vaccine is 2 doses with an interval of 14 to 28 days; each human dose is 0.5 ml.
It is reported that in Brazil, the vaccine has a protective effect of 100.00% in hospitalized, serious and dead cases, and has a protective effect of 83.70% in new coronary cases with obvious symptoms and that require medical intervention, including those mild cases that do not. require medical intervention. The protective effect of all new crowns in China is 50.65%, in Turkey, the protective effect of preventing new corona virus infection is 91.25%.
After obtaining the vaccine protection efficacy results, Kexing Zhongwei formed a formal Phase III clinical investigational drug in accordance with the requirements of the State Drug Administration.reportSubmit CDE and submit a conditional request for quotation by February 3. Conditional approval for listing this time is based on the two-month results of Creativity’s overseas Phase III clinical protection efficacy trial. The final analysis data has not yet been obtained, and the effectiveness and safety results have not yet been confirmed.
Injector manufacturer’s phone call was interrupted
At present, the annual production capacity of the new corona vaccine from Sinopharm and Kexing has expanded to 2 billion doses. Currently, a new vaccination crown nationwide.the playThe process is proceeding in an orderly manner: the demand for syringes for vaccine production has increased significantly, the production capacity of the relevant manufacturers is low, and prices have also increased.
Sanxin MedicalviceGeneral manager, Chief Financial Officer Le Zhenrong told a reporter for China Securities News that the company used to sell syringes at a price between 0.18 and 0.2 yuan, but now it has risen to around 0.3 yuan in China, a 50% increase. Due to the increase in demand for syringes, the company’s order calls have now exploded and new orders have continued to rise. Currently, orders are full, but production capacity is limited and some orders cannot be received. The company is currently purchasing equipment to expand production and plans to double its production capacity in May. The company has been working overtime to produce and plans to take just two days off during the Spring Festival.
WanbondAccording to the report, the current price of the company’s syringes has increased from about RMB 0.1-0.15 per syringe in the past to over RMB 0.3. The company recentlyinvestmentPersoninteractiveDepending on the platform, the companyCompany of the sunKangkang Medical small capacity syringes of 1 ml or less can be used for vaccination (including new corona vaccine). Start syringe late 2020international marketDemand has increased rapidly and the scale of Kangkang’s medical orders has risen from one million before the new corona epidemic to ten million. At present, according to the current production capacity of Kangkang Medical, the available orders have been placed until May 2021, and the production capacity of small volume syringes has been in full production. The company has organized the production of overtime during the Spring Festival to digest current orders.At the same time, in response tomarketLawsuit, the company has accelerated China-Africa medical technologyindustryThe construction of the syringe production line in the park is planned to expand the production capacity by approximately 4 times in the second quarter of 2021.
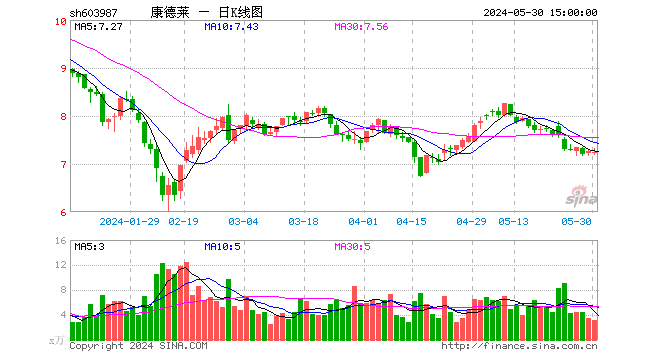
CondeletThe share price rose 21% in three days
In the A share market, the syringe concept stocks includeAkeli、Wanbond、Sanxin Medical、Chutian technology、East Fulong、Condelet、Super clean tianhuaWait.
On February 5, syringe-related stocks rose overall, among which Kangdelai’s share price rose 21.12% for three consecutive days.
CondeletadIt shows that the company’s core business is medical lancing devices, of which small vaccine-related syringes represent a relatively small proportion. In 2019, sales of small-size syringes accounted forOperating incomeAbout 5%.
New crown seedlingsIndustrial chainBefore packaging the vaccine andRaw Materials, Mainly related to medical glass vials, pre-filled syringes, vial caps, pharmaceutical excipients, etc .; midstream is the development and production of vaccines, mainly related to the development and production of related vaccinescompany; Downstream is the end-use of vaccines and vaccine treatment, which mainly involves the treatment of syringes and medical waste. Yuekai Securities believes that considering the continued listing of the new vaccine from the crown and abroadDepartureFactors, the injection needle / syringe market will usher in a substantial expansion in 2021.

Massive information, accurate interpretation, all in the Sina Finance APP
Editor in charge: Peng Jiabing