[ad_1]
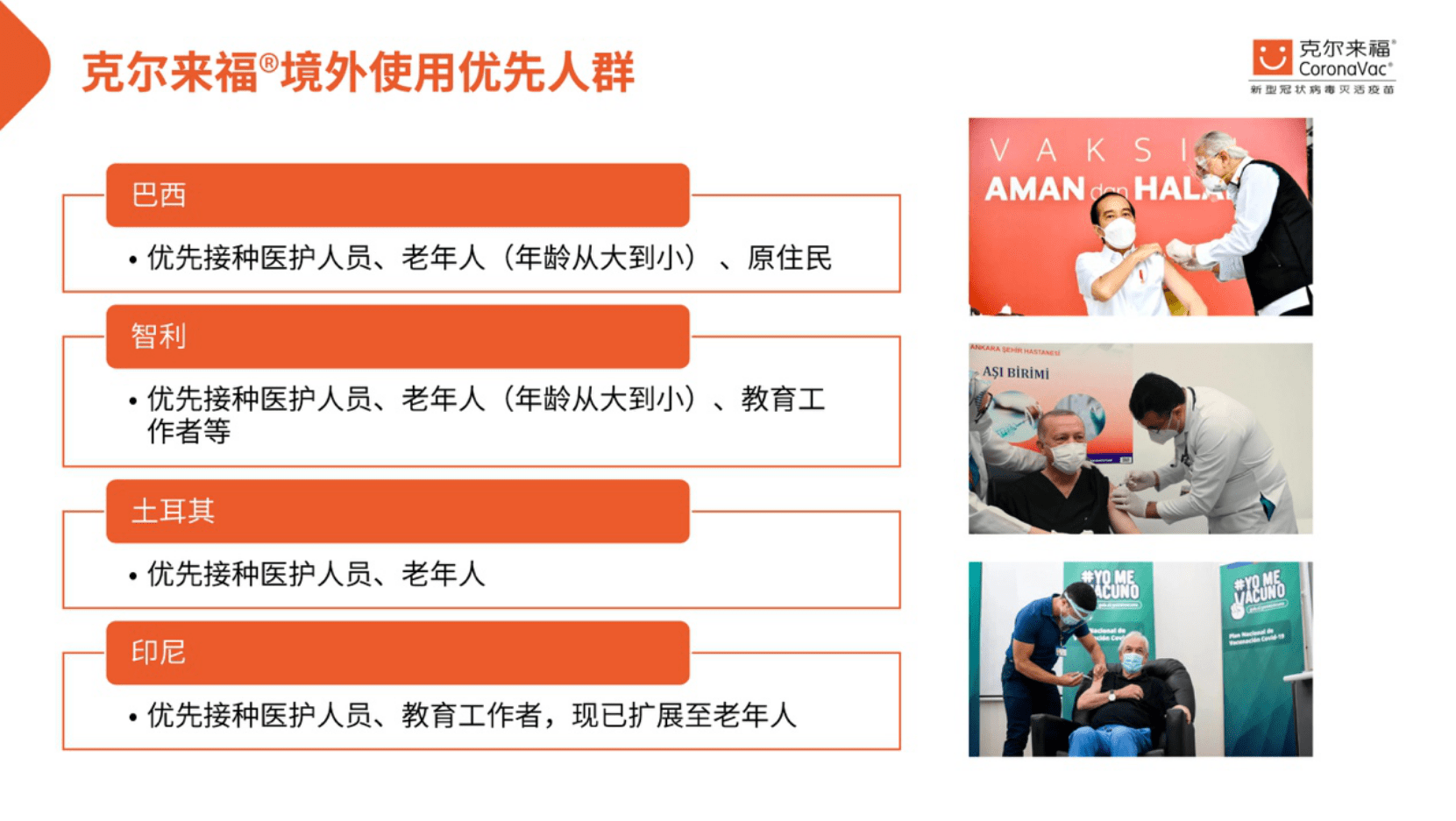
The Beijing News (Reporter Dai Xuan) The new corona vaccine developed by my country has been put into use in more and more countries. The reporter learned from Beijing Kexing Zhongwei Biotechnology Co., Ltd. that since December last year, the new inactivated corona Kellyf vaccine has been approved for emergency use in more than 20 countries and regions, including Indonesia, Turkey, Brazil, Thailand and Hong Kong. Brazil, Indonesia, Turkey, Chile, and other countries have included the elderly and other high-risk populations as priority vaccines for key populations.
Kerrlive Vaccine Data Map Provided by Kexing Zhongwei
The elderly can produce certain neutralizing antibodies after vaccination.
On February 5, the National Medical Products Administration approved the new coronavirus inactivated vaccine developed by Beijing Kexing Zhongwei Biotechnology Co., Ltd. for conditional marketing in China. The vaccine is suitable for the vaccination of people over 18 years of age to prevent diseases caused by a new coronavirus infection. The basic immunization schedule for the vaccine is 2 doses with an interval of 14 to 28 days; each human dose is 0.5 ml.
In June 2020, Kerlaifu was the first to be approved for emergency use in China and, since July, it has been carrying out emergency use for specific groups of people in China. Since December 2020, more than 20 countries and regions including Indonesia, Turkey, Brazil, Chile, United Arab Emirates, Colombia, Thailand, Uruguay, Philippines, Malaysia, Hong Kong, etc. have successively approved Clareford emergency use in the local area. Kexing Zhongwei’s new crown vaccine production quality management system has passed GMP inspections in China, Brazil, Indonesia, Chile and other countries.
According to the company, among the many countries that have approved the emergency use of Kellyford, Brazil, Indonesia, Turkey, Chile and other countries have included the elderly and other high-risk groups as priority vaccines for key populations. Existing clinical trial data show that people 60 years of age and older produce some degree of neutralizing antibodies after vaccination. When vaccines are used by relevant agencies for disease prevention and control, the need for vaccination needs to be evaluated in light of the health status and exposure risks of people 60 years of age and older.
A total of 25,000 people enrolled in the phase III clinical study had no serious adverse effects related to the vaccine.
It is understood that Kerlaifu is inoculated with the new coronavirus (strain CZ02) in African green monkey kidney cells, which are obtained by cultivating, collecting the virus liquid, inactivating the virus, concentrating, purifying and adsorbing on aluminum hydroxide, without preservatives. .
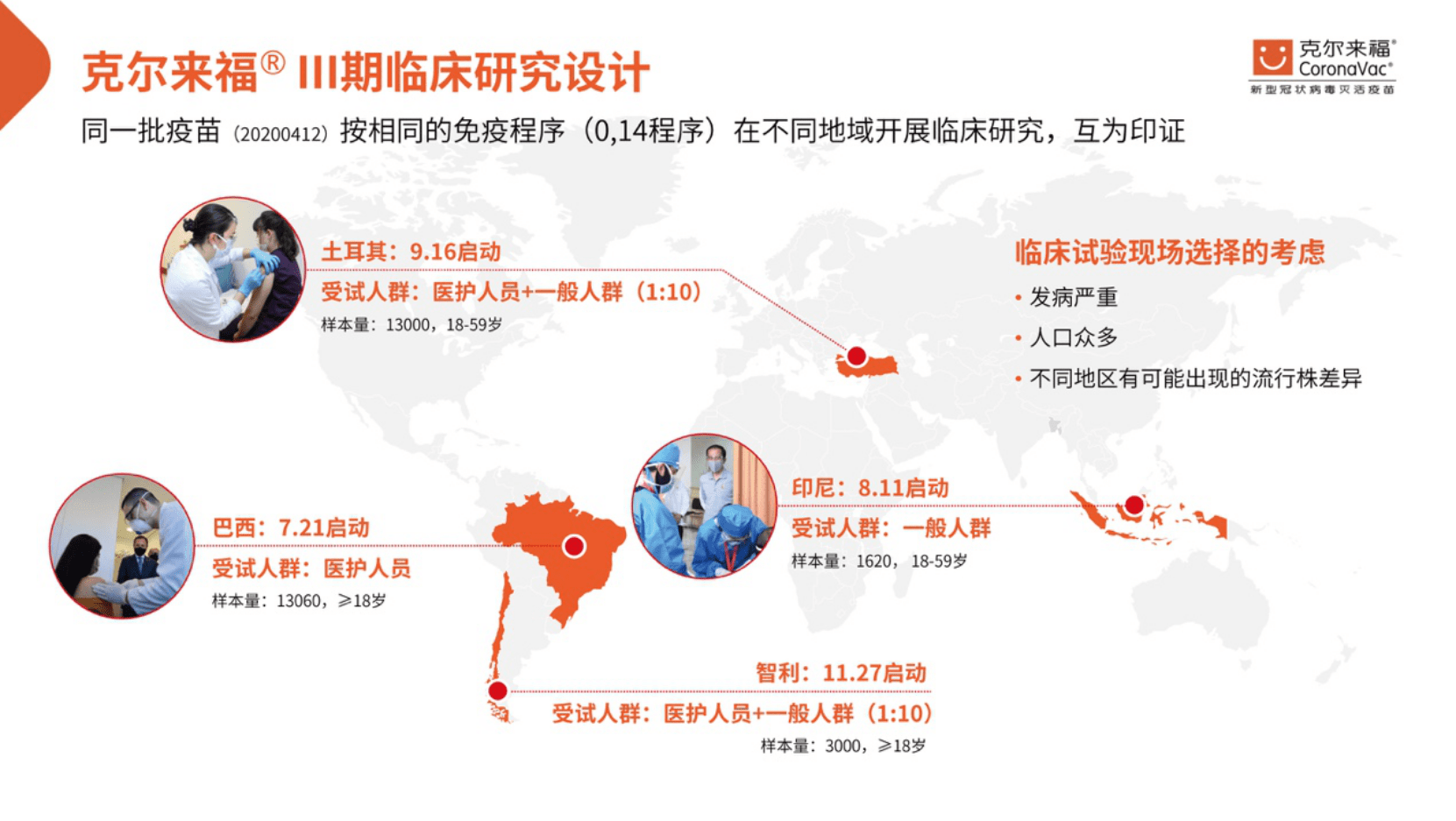
In the early stage, Kerlaifu went through rigorous animal experiments and phase I and II clinical studies. The research data showed that the vaccine has good safety and immunogenicity. Since July 21, 2020, Coxing Zhongwei has successively selected Brazil and Chile in South America, Indonesia in Southeast Asia, and Turkey in the Middle East to conduct Phase III clinical studies in four countries with different regions and characteristics. Global clinical research design. These countries have designed different clinical research programs according to their respective conditions, with a total of 25,000 people enrolled. Based on current monitoring data analysis, the vaccine is safe and no serious vaccine-related adverse events have been found as judged by the investigator.
Kerrlive Vaccine Data Map Provided by Kexing Zhongwei
Phase III clinical studies conducted in Brazil and Turkey respectively evaluated the protective efficacy of Kellyford in high-risk groups (medical personnel receiving COVID-19 patients) and the general population.
Turkey’s analysis based on 29 cases showed that the protective effect of preventing COVID-19 after 14 days of vaccination with two doses of the vaccine in the 0.14 day schedule was 91.25%.
The protective efficacy of Brazil after the 0.14-day program to vaccinate two doses of vaccine 14 days after the prevention of the disease caused by the new coronavirus (COVID-19) is: 100.00% for the protection of hospitalized, grave and dead. cases and for patients with obvious symptoms The protective effect of new coronary cases requiring medical intervention is 83.70% and the protective effect of all new coronary cases, including mild cases that do not require medical intervention, is 50, 65%.
The first new corona vaccine production line that Kexing Zhongwei has built and put into operation at the end of August 2020 has an annual production capacity of 500 million doses. In February this year, Kexing Zhongwei completed the construction of the second production line and put it into operation, and the annual production capacity of Kerlaifu stock solution was increased to more than one billion doses.
In terms of international cold chain distribution, the company has achieved strategic cooperation with many national and international airlines and temperature controlled medical air transport solution providers, forming a cold chain transportation assurance program with a broader coverage and stricter standards. The availability of vaccines in many countries and regions offers guarantees.
Beijing News reporter Dai Xuan
Editor Liu Mengjie reviewing Li LijunReturn to Sohu to see more
Editor:
Disclaimer: The opinions in this article only represent the author himself. Sohu is an information publishing platform. Sohu only provides storage space services.
[ad_2]