[ad_1]
Original Title: China’s First Registration Certificate for Early Cancer Detection Approved for Screening People Aged 40 to 74 at High Risk of Colorectal Cancer
China Net Finance, Nov 26 (Reporter Du Ding) Yesterday, at the press conference of “Yinuo advances China’s first cancer screening certificate,” Nuohui Health announced that the National Medical Products Administration (NMPA) approved the The rectal cancer early detection product is Chang Weiqing’s innovative three-class medical device registration app, and it is clear that Chang Weiqing is suitable for screening “40-74-year-olds at high risk of colorectal cancer “in its intended use.
This is reported to be China’s first and only registration certificate for early cancer detection products approved by the State Food and Drug Administration. Currently, Chang Weiqing’s “In Vitro Diagnostic Reagent Product Registration Technical Review Report” has been published in its entirety on the official website of the State Administration of Medicines.
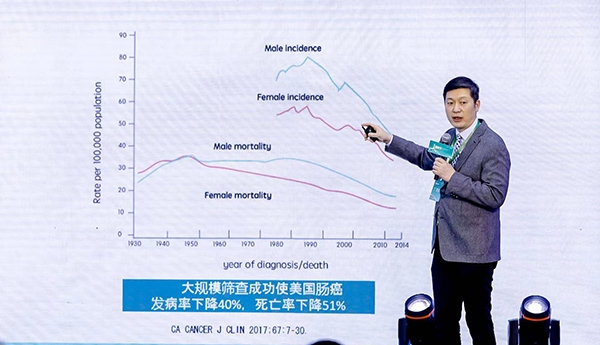
According to the data, Chang Weiqing is China’s first and only cancer early detection product that has been verified by large-scale multicenter registry prospective clinical trials. “Clear-C” was launched in September 2018. It lasted 16 months and a total of 5,881 cases were registered. It was carried out jointly by 8 large tertiary hospitals across the country and provided the highest level of scientific evidence for verification of screening performance. Based on the results of the Clear-C clinical trial, Chang Weiqing’s detection sensitivity for colorectal cancer is 95.5%; for advanced adenomas, the detection sensitivity is 63.5%, which is more than double that of traditional stool occult blood detection; for colorectal cancer The negative predictive value of is as high as 99.6%.
 Professor Ding Kefeng
Professor Ding Kefeng“Clear-C” Clinical Research Project Leader, Vice Dean of the Second Affiliated Hospital of Zhejiang University School of Medicine, Chair-designate of the Colorectal Cancer Committee of the China Cancer Association, and a colorectal cancer cohort study in the 13th National Five-Year Plan Chief Scientist Professor Ding Kefeng said that this experiment has three characteristics. First, China had never before had a prospective clinical trial registered for the early detection of bowel cancer. Second, the sample size is large, and third, the excellent test results. ‘All enrollees must complete the colonoscopy at the same time as the Chang Weiqing test, and compare the gold standard of colonoscopy and pathology to verify the results of the Chang Weiqing screening. The large-scale prospective trial design’ Clear C ‘is close to screening. The real application scenarios of AIDS are conducive to early detection, with clear clinical importance, which can greatly enhance the effect of early treatment and prevent the occurrence of intestinal cancer. value for the detection of malignant tumors in China.
On the same day, Nuohui Health Chief Scientist Dr. Chen Yiyou, on behalf of the company, donated 1 million yuan to the Zheng Shu Cancer Development Fund of Zhejiang University Education Foundation School of Medicine to pay tribute to 90-year-old Zheng Shu “China’s first colorectal cancer early detection person.” professor. The donation will be determined by Professor Zheng Shu, who will invest in training and academic research on colorectal cancer.
–dialogue:
Zhu Yeqing: Will Establish More Projects In The Field Of Early Cancer Detection

reporter:According to the data, “Clear-C” was launched in September 2018. It took 16 months and a total of 5,881 cases were enrolled. How was the number of 5,881 cases selected? What groups are you targeting?
Zhu Yeqing:The Drug Administration’s clinical trial protocol has very strict regulations. First, your future applicable population is the population that enters the group. The specific specifications are defined according to the “Expert Consensus on Early Diagnosis and Detection Strategies for Colorectal Cancer in China” issued by the Professional Committee for Colorectal Cancer of the China Cancer Association in October 2018. The first is that they are over 40 years old; the second is that they have some habitual lifestyle habits or a family history, such as some cancer in their immediate relatives, such as diabetic patients, but they cannot have a colonoscopy in the last five years.
reporter:What is the market positioning of Chang Weiqing? The current price?
Zhu Yeqing:The current market price is 1996 yuan. Hope all the designs for this product are based on home sampling. SF Express, JD.com and EMS can send samples to our central laboratory for analysis. After submitting the sample, we go through a very standard and standardized process, and the report can be issued within five days, and the report can be viewed on the user’s mobile phone.
All of our products are easily accessible to users. Going forward, Nuova hopes to make better use of the capital platform to further expand the market development of our approved products. At the same time, we will also implement more channels in the field of early cancer detection. Our gastric cancer clinical data has been submitted to the National Food and Drug Administration and is expected to be approved next year. The cervical cancer product will undergo a prospective experiment next year. This experiment is very big. The registered population will be about 3-5 million. It takes three to five years.
reporter:If our products are mainly home-based, will we mainly develop channels based on medical examination institutions or create a sales platform?
Zhu Yeqing:If the product is recognized for the first time by the public, it must be endorsed by a doctor, so the endorsement of the doctor is very important. I hope that the doctor channel can establish a high degree of brand professionalism, and we will definitely cooperate with more partners, such as insurance and Internet medical services. For the advancing medical market, we will create our own team and partners to advance together.

Massive information, accurate interpretation, all in the Sina Finance APP
Editor in charge: Zhang Mei