[ad_1]
Original title: Cansino’s new crown vaccine has a single injection with a severe protection rate of 90.98%! Vaccine concept stocks collectively exploded
On February 10, vaccine stocks continued to rise. Cansino AH shares rose sharply. Among them, Cansino A shares rose above the daily limit in the afternoon and H sharesLift upIt went up more than 14%. For A shares,Kangtai Bio、Watson Bio、Kehua Bio、ZhifeiExpect a substantial increase. In the news, the single dose of Cansino’s new crown vaccine has a severe protection rate of 90.98%.
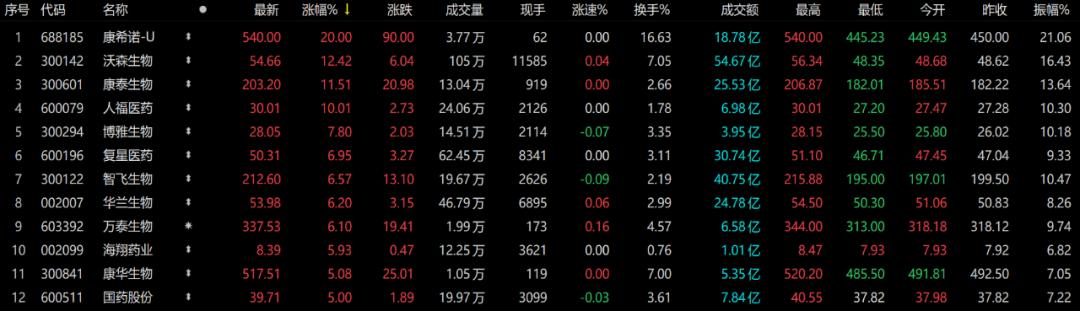
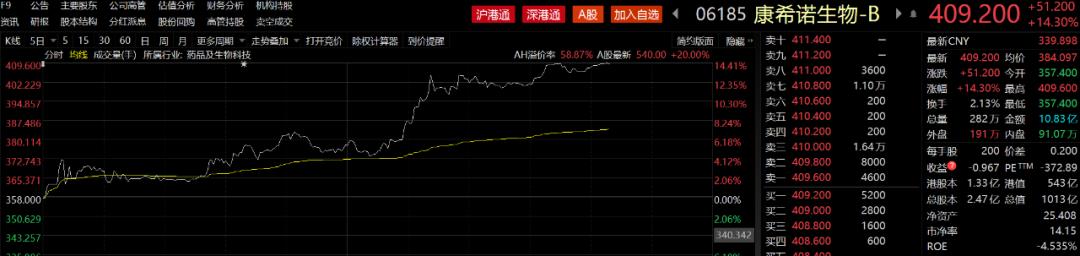
source:Choice
The new Cansino crown vaccine has a severe protection rate of 90.98%
According to the website of the State Supervisory Commission of the Central Commission for Disciplinary Inspection, Chen Wei, an academic at the Chinese Academy of Engineering and a researcher at the Academy of Military Medicine of the Academy of Military SciencesequipmentCanSino BioCooperative research and developmentThe latest data on the Ad5-nCoV adenovirus vector vaccine have recently been published. Results from the interim analysis of the phase III clinical trial of Ad5-nCoV in Pakistan showed that the protective effect of single vaccination against new severe coronary pneumonia was 100% and the overall protective effect was 74.8%.
Ad5-nCoV is currently in phase III clinical trials in countries such as Mexico, Russia, Pakistan, Argentina and Chile.CanSino Bio launched on February 1adAccording to the report, the phase III clinical trial of Ad5-nCoV has completed the vaccination of more than 40,000 subjects in 78 clinical research centers in 5 countries. Based on global test data, 28 days after a single injection, the vaccine has a 90.98% protective effect against new severe coronary pneumonia and an overall protective effect of 65.7%.

Source: Website of the Central Commission for Disciplinary Inspection and the State Supervision Commission
Currently, three new corona vaccines in my country have announced their phase III clinical protective effects. In addition to Cansino, Kexing Holdings Biotechnology Co., Ltd.the companyIt announced the Phase III clinical data of the new coronavirus inactivated vaccine developed by its subsidiary Xingzhongwei, showing that in Brazil, Kellyford’s protective effect in hospitalized, severe and fatal cases is 100%, and it is against obvious symptoms and the protective effect of new cases of coronavirus that require medical intervention is 83.70%, and the protective effect of all new cases of coronavirus, including mild cases that do not require medical intervention, is 50.65%; In Turkey, the protective effect of preventing new coronavirus infection is 91.25%.
In addition, the Sinopharm Beijing Biology official website previously announced the results of the interim analysis data from the phase III clinical trial of the new inactivated coronavirus vaccine. All vaccinated individuals produced high antibody titers, the neutralizing antibody positive conversion rate was 99.52%, and the protective efficacy of the vaccine against diseases caused by new coronavirus infection was 79.34%.
The production capacity of the two vaccines will exceed 2,000 million doses
Based on the two-month results of Kerrlive’s overseas phase III clinical protection efficacy trial, the State Food and Drug Administration approved the conditional listing of Kerrlive in China on February 5 in accordance with the law. This is also the second new corona vaccine approved for sale in China after the Sinopharm Group.
In terms of production capacity, Coxing Zhongwei is reported to have built the first new corona vaccine stock solution production line that will be put into operation at the end of August 2020.Production capacityUp to 500 million doses. Kexing Zhongwei has completed the construction of the second drug production line, which is expected to increase the annual production capacity of Kerlaifu drug solution to more than 1 billion doses after it is put into use in February this year. . At present, the company is further accelerating the construction of the raw liquid vaccine and finished product production capacity, and is striving to match the filling and packaging capacity with the production capacity of raw liquid. Sinopharm also previously stated that planned vaccine production capacity will reach 1 billion doses by 2021.
National Health Commissionnews spokespersonMi Feng previously introduced that as of 24:00 on February 3, the national accumulatedreportKey populations received 31.236 million doses of the new coronavirus vaccine.
Also, other household vaccinescompanyIt is also stepping up research and development for the new corona vaccine. The relevant head of the Ministry of Science and Technology presented at the first China Hematology Development Conference on January 30 that currently 16 new coronavirus vaccines have entered the clinical trial stage in my country, of which 7 have entered in the phase III clinical trial stage.
Many new corona vaccine research and development companies are also actively expanding their production capacity. December 27, 2020Watson BioNew coronavirus vaccine base installed in Beijing Daxing BiomedicalindustryThe park will be mass-produced in mid-2021 and is expected to produce 200 million doses of vaccines annually. Yu Xuefeng, President and CEO of Cansino Bio, previously stated that the company is using the existing facilities to produce vaccines for trials. clinical and to meet some urgent needs; new facilities are being built toWarrantyIn 2021, more than 200 million doses of vaccine can be produced.
Major vaccine companies are expected to benefit fromIndustrial modernization
Everbright ValuesThe new vaccine is believed to crownIndustrial beltCome lead a lot of action.short termLook at the vaccineIndustrial chainThe company is expected to be straightto acceptSuitable for the progress of the development and vaccination of the new corona vaccine; in the medium and long term, the new corona vaccineIndustrializationThe company’s attention has risen to unprecedented heights. Policies and rules that may be introduced in the future that are beneficial to the vaccine industry deserve attention: such as the vaccine reservation system, updates to the technology of vaccines and the promotion of vaccination.Support forPolicies etc As the new corona pneumonia epidemic is still spreading around the world, countries around the world still have increased pressure for prevention and control, and the new corona vaccine will be it has become a rigid necessity for countries to respond to the epidemic.productSome national new crown vaccine development companies with advanced advancements and sufficient production capacity are expected to benefit from the demand. In general, the major vaccine companies with great prosperity and large varieties are expected to benefit from the industrial modernization catalyzed by the epidemic.
China valuesHe said that my country’s multiple technical routes of new corona vaccines have entered the implementation period one after another, which is expected to boost related companies.PerformanceHighly elastic growth, continue to recommend the objectives of the vaccine sector.
(Source: China Securities Journal)
(Responsible editor: DF537)
I solemnly declare: The purpose of this information disclosed by Oriental Fortune.com is to spread more information and has nothing to do with this booth.
[ad_2]