
[ad_1]
Original title: Pfizer vaccine in the United States will begin on the 14th, priority will be given to medical personnel
According to Reuters, as Pfizer and BioNTech plan to supply the new corona vaccine to the countries most affected by the epidemic starting on the 13th, the US “Temporal Movement” plan (for the US government) Army Gustave Perna, who initiated the development and supply of the new corona vaccine, said that the first injection of the vaccine in the United States will be injected on the 14th.
 Gustav Pana
Gustav PanaPana told a news conference on the 12th that medical personnel and the elderly in long-term care facilities are expected to be the main vaccinators in the first wave of 2.9 million vaccines this month. Medical staff will begin vaccinating on the 14th. Vaccination work for nursing home residents will begin next weekend. Pana also said that despite months of preparations, the distribution and administration of vaccines to up to 330 million vaccine recipients still represents a great logistical challenge. The transportation requirements for this vaccine are reported to be very complicated and should be stored at -70 degrees Celsius.
Pana said the vaccine developed by Pfizer and its German partner Biotech will be delivered to 145 locations in the United States on the 14th, and the remaining 636 selected vaccination points in various states and regions will be delivered in 15. The vaccines will be received on 16 January. He also added that Pfizer will have more doses of vaccine available to distribute and ration each week. He also said that within three weeks, the “surge” program should allow Pfizer’s vaccine to be distributed to all medical institutions in the United States.
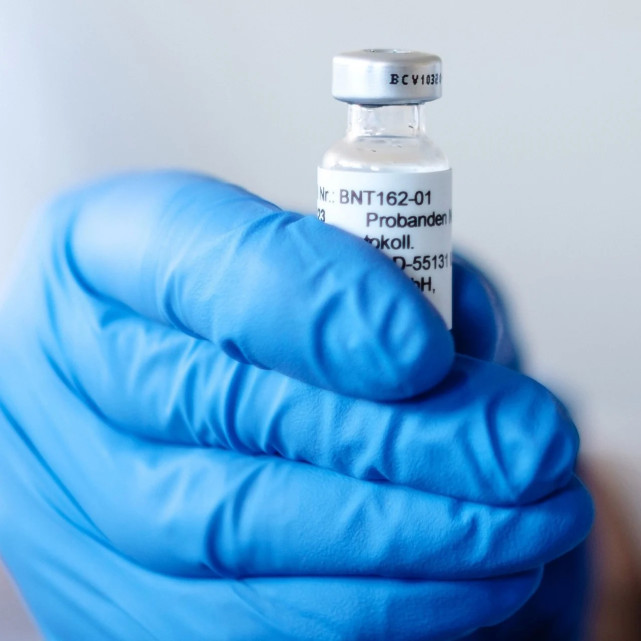 BNT162 new corona vaccine developed by Pfizer and Biotech
BNT162 new corona vaccine developed by Pfizer and BiotechThe US Food and Drug Administration (FDA) approved the emergency use authorization of the Pfizer vaccine on the night of the 12th. In subsequent trials, the vaccine has a 95% effective rate in preventing new pneumonia coronary. In the United States, the number of confirmed cases of new coronary pneumonia has exploded, thousands of people die every day, and the capacity of intensive care units in hospitals in the United States is nearly saturated. Currently, more than 295,000 Americans have died of a new coronary pneumonia.
With the imminent large-scale distribution and vaccination of Pfizer’s new corona vaccine in the United States, FDA executives sought to assure Americans that this record-breaking speed of the vaccine was justified and did not sacrifice safety. FDA Commissioner Stephen Hahn said: “We are working quickly based on the urgency of this pandemic, not due to any other external pressure.”
 The British are vaccinated
The British are vaccinatedThe Pfizer vaccine is the first batch of new coronavirus vaccines approved for mass vaccination in the United States, and before the United States, United Kingdom, Canada and three other countries have also approved the emergency use of this vaccine. Among them, the UK started mass vaccination of this vaccine for the public on day 8, but on the first day of vaccination, 2 people had adverse reactions. The British Medicines and Health Products Regulation Agency (MHRA) subsequently issued a warning that people with a history of “severe” allergic reactions should not receive this new corona vaccine. In addition, the FDA reviewed the vaccine before approving the emergency use authorization for the vaccine and found that four participants in the phase 3 trial who received Pfizer’s new corona vaccine developed Bell’s palsy (a temporary facial paralysis), although the symptoms are usually disappeared on its own, but it is unclear what caused this situation. The FDA said that despite reports that two people in the UK with a history of severe allergies had serious adverse reactions after receiving the Pfizer vaccine, the vaccine is safe for most allergic Americans.