[ad_1]
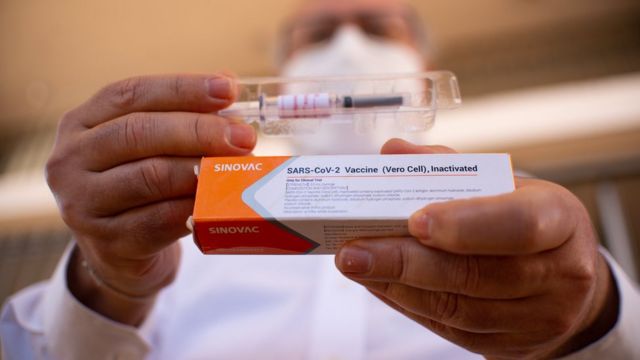
Image source,fake images
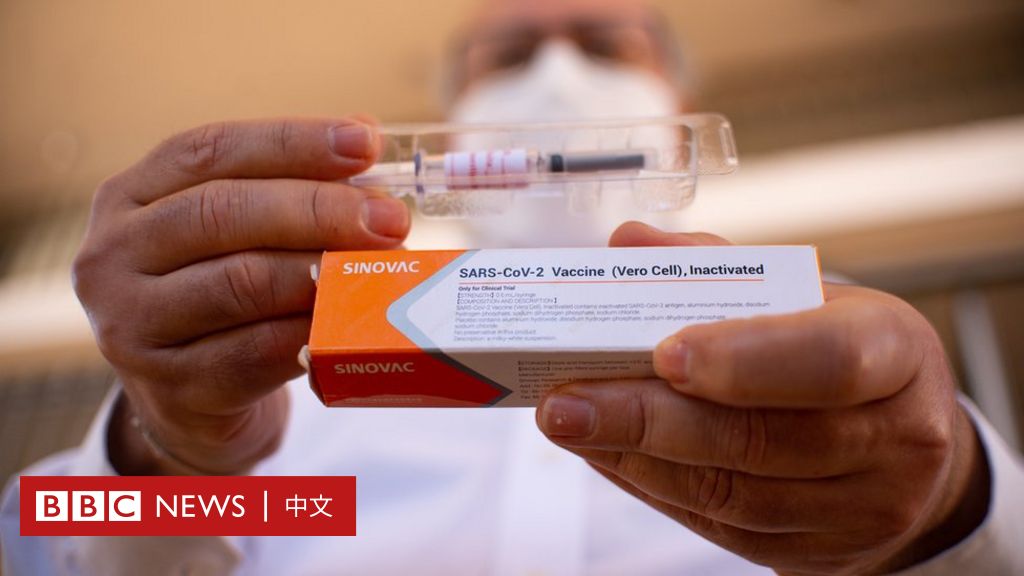
The vaccine developed by Kexing Biological is one of more than a dozen vaccines in the final testing stage in the world, this stage is called the third-stage trial.
Brazilian authorities said a volunteer had a serious adverse reaction. Two days after the suspension of vaccine clinical trials in China, they announced the resumption of trials on Wednesday (November 11).
Previously, the Brazilian health oversight department did not disclose details of the adverse reaction, but international media reports indicated that the volunteer “died by suicide.” The head of the research institute that carried out the experiment said that death has nothing to do with the vaccine.
The Brazilian Health Inspection Agency said in a statement Wednesday that there is now enough information to resume vaccine trials. “It is important to clarify that the suspension (test) does not necessarily mean that there is a problem with the quality, safety or effectiveness of the product under investigation,” the statement said.
This vaccine is developed by China Kexing Biological. Kexing Biotechnology responded on Tuesday (Nov 10), pointing to relevant media reports, but said it was confident in the safety of the vaccine.
Why is the trial suspended?
The Brazilian Sanitary Inspection Agency said on Monday (November 9) that after a serious adverse reaction occurred in volunteers, it decided to suspend clinical trials of the Kexing Biovaccine.
The Brazilian Sanitary Inspection Agency did not reveal what happened. Last-stage trials of Kexing’s biological vaccine are still ongoing in Indonesia and Turkey. Neither country has announced a suspension of the trials.
According to Reuters, Indonesia’s state-owned biopharmaceutical company Bio Farma said Tuesday that its trial of the Koxing vaccine was “progressing smoothly.”
Image source,EPA / WU HONG
The CoronaVac vaccine was jointly developed by the Beijing Kexing Biological Research Institute and Butantan.
Dimas Covas, director of the Butantan Institute in Brazil, which conducts vaccine trials, told the media that the suspension of the trial was related to a case of death, but that the death was not related to the vaccine.
This statement was supported by the Minister of Health of the State of São Paulo, Jean Gorinchteyn, who stated at a press conference that the death was an “external incident” and had nothing to do with the vaccine.
Kovas said there were no adverse reactions to the vaccine. “We believe that the decision of the Brazilian Sanitary Inspection is very strange because it has nothing to do with vaccines. Currently there are more than 10,000 volunteers,” Covas told Brazilian television station TV Cultura.
Reuters reported that the Sao Paulo state government, which is conducting vaccine trials, said a volunteer had died and that the cause of death was recorded as suicide, and that it is currently under investigation. Reuters said it had seen a police report.
A police report obtained by The New York Times also showed that the volunteer died on October 29 and is currently under investigation for suicide.
Covas also said the suspension of the test caused “outrage.” Kovas also told a news conference Tuesday that he hopes to resume the experiment as soon as possible. It is not uncommon to suspend clinical trials.
In September, after a participant had an adverse reaction, the UK suspended the trial of the new coronavirus vaccine from AstraZeneca and the University of Oxford, but then restarted the trial.
Image source,Government of São Paulo / Reuters
Doria (left) was an ally of Bolsonaro (right) but later became his main critic.
How did the parties respond?
Brazilian President Jair Bolsonaro has long been critical of Chinese vaccines, saying that Brazil will not buy such vaccines. He has a political fight with the governor of Sao Paulo, João Doria. Doria has always supported vaccine trials.
Bolsonaro said in late October that the Brazilian government would not buy the new corona vaccine made in China and said that the Brazilian people would not become anyone’s mice.
Bolsonaro declared on Facebook that stopping the vaccine trial was “another Bolsonaro victory.”
This vaccine is developed by China Kexing Biological. Kexing Biotechnology responded on Tuesday (November 10), pointing to relevant media reports, but said it was confident in the safety of the vaccine.
“After communicating with the Brazilian partner Instituto Butantan, the head of the institute believes that this incident has nothing to do with the vaccine. Kexing will continue to communicate with Brazil on this issue. Clinical research in Brazil will continue to carry out related work in strict compliance with the requirements of the GCP “. Kexing Biological responded.
Image source,fake images
Doria had previously actively promoted the China Kexing experimental vaccine and announced plans to use it to vaccinate São Paulo residents.
According to data from Johns Hopkins University, Brazil is one of the countries most affected by the new corona virus, with more than 5.69 million confirmed cases, only surpassed by the United States and India, ranking third in the world, with more of 160,000 deaths.
World vaccine progress
The vaccine developed by Kexing Biological is one of more than a dozen vaccines in the final stage of testing in the world, a stage called the third phase of the trial. Phase III trials are a critical period for vaccine development and some vaccines may fail at this time.
Before the suspension of the Kexing vaccine trial in Brazil, the American pharmaceutical multinational Pfizer announced that the new corona vaccine developed by the company and the German pharmaceutical company BioNTech has an efficiency of 90%.
In addition to conducting phase III clinical trials abroad, China has also implemented trial vaccination of the new corona vaccine in China.
Kexing Biotech previously stated that nearly all employees and their families have been vaccinated. A Chinese health official previously said that no serious side effects were seen in clinical trials.