
[ad_1]
Original title: The new corona vaccine made in China is presented in the box with a lot of information
Han Shengjiang paper
The new inactivated corona vaccine, which has received much attention, made its public debut at the 2020 China International Trade in Services Fair, making the Fair’s Epidemic Prevention and Public Health Zone a star booth for several consecutive days.
On September 7, The Paper reporters saw in the exhibition area, including the Beijing Institute of Biological Products, the Wuhan Institute of Biological Products, and Kexing Holding Biotechnology Co., Ltd. (abbreviated as: Kexing) all three Inactivated vaccines for the new coronavirus are on display.
The journalist saw on the outer packaging of the three vaccines exposed during the service and the trade fair, the vaccine specifications are 0.5 ml each, and the vaccination route is intramuscular injection into the deltoid muscle of the part upper arm.
In terms of doses: the vaccine package from the Wuhan Institute of Biology of China shows: “2-3 basic immunization doses, the interval between the first and the second dose is 2-4 weeks, and the interval between the second and the third dose is 4 weeks. ” Vaccine packaging from Beijing Institute of China Biology Display: “Basic immunization is 2 doses, with a 2-4 week interval between each dose.” The Kexing vaccine package shows: “The basic immunization schedule for emergency vaccination is 2 doses, with an interval of 2 weeks. For basic immunization for routine vaccination The procedure is 2 doses, with one month Of diference “.

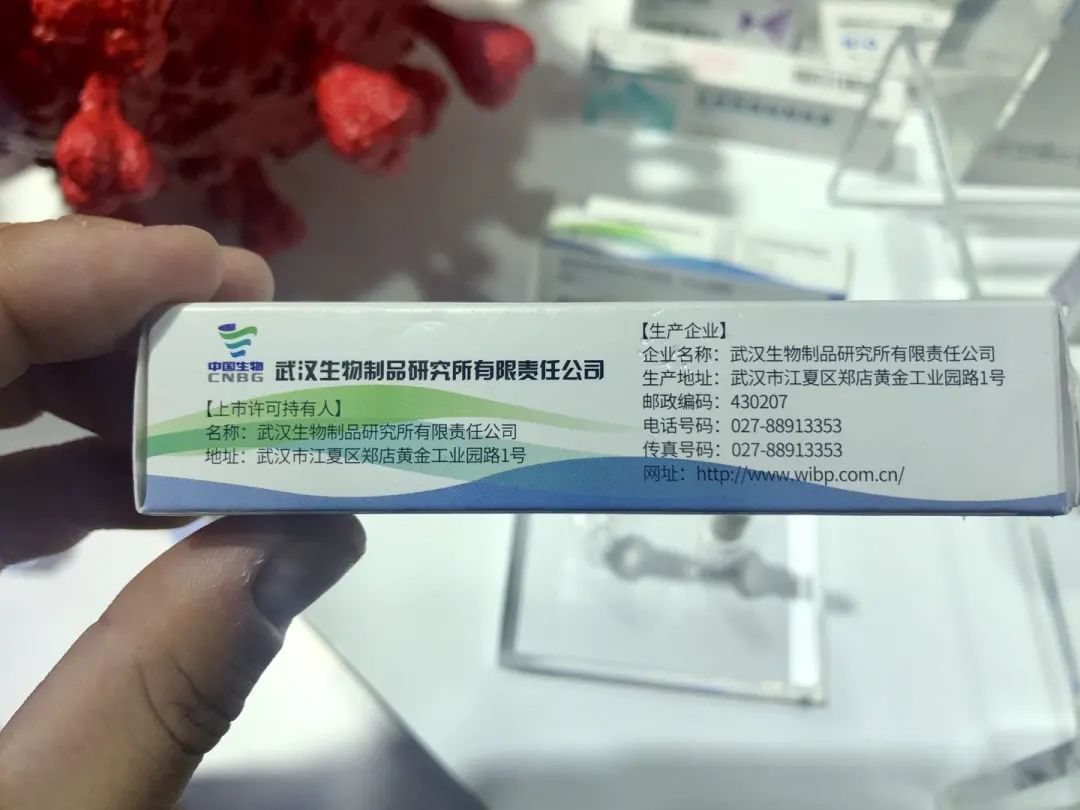


China Institute of Biology Wuhan Vaccine Institute The images in this article are all from The Paper, a reporter from Shengjiang Han
According to the vaccine package of the Wuhan Institute of Biology of China, the target of the vaccine is “applicable to susceptible persons aged 6 years and older”, and the validity period is tentatively set at 36 months.
The vaccine package from China’s Wuhan Institute of Biology also showed that the active ingredient in the product is the inactivated antigen of the new coronavirus strain WIV04, and each vaccine contains 200 WU of antigen. The Kexing vaccine package shows that each dose of the vaccine contains 600SU of the new coronavirus antigen. (WU and SU are custom units of the two companies)
According to the data, the new coronavirus strain WIV04 was successfully isolated by Shi Zhengli and others from the Institute of Virology at the Wuhan Institute of the Chinese Academy of Sciences. Kexing uses the independently isolated and named “CZ” strain.


Beijing Vaccine Institute
The three new corona vaccines mentioned above are understood to have been included in the national emergency vaccination program for high-risk exposed populations. According to the Joint Prevention and Control Mechanism of the State Council, China approved the “Emergency Use Plan (Test) of the New Coronavirus Vaccine” on June 24 and officially launched the emergency use of the new coronavirus vaccine. coronavirus on July 22.
According to Zheng Zhongwei, director of the Science and Technology Development Center of the National Health Commission, in accordance with relevant laws and regulations, emergency (trial) use is limited to specific populations that have high risks of exposure and cannot use current effective protection measures. Special groups such as medical personnel, epidemic prevention personnel, border inspection personnel, and personnel who ensure basic urban operations are the target of emergency vaccination.
According to the Joint Prevention and Control Mechanism of the State Council, there are currently four new coronavirus vaccines in China that have started international phase III clinical trials. In addition to the above three vaccines, there is also a new recombinant adenovirus vector vaccine for the new coronavirus developed by Chen Wei’s team from the Academy of Military Medicine of the Academy of Military Sciences.
Kexing field staff told reporters that the company’s vaccine is currently in phase III clinical trials in Brazil, Indonesia and other countries. Chinese biological field staff said that after conducting the first and second phase of clinical trials in China, given that the country is no longer a high incidence epidemic area, they have turned abroad, successively to the United Arab Emirates, Bahrain , Peru, Morocco, Argentina, Many countries, including Jordan, have conducted phase III clinical trials and are currently awaiting data.




New Kexing Crown vaccine
In response to the adverse reactions of the injectable vaccine, the vaccine package from the Beijing Institute of Biology of China showed: “Generally, within 24 hours after vaccination, injection ministries may experience pain, tenderness, redness and itching, and in most cases they go away in 2-3 days. ” The other two Vaccine is marked as “see instructions for details”.
However, Kexing staff at the site told reporters that current clinical data shows that the main adverse reactions observed are local pain at the injection site, followed by fatigue, local swelling, etc., all of which are transient and only a few fevers are observed. And allergic reactions, no serious adverse reactions, indicating that the vaccine is safe.
New Kexing Crown vaccine
In response to the adverse reactions of the injectable vaccine, the vaccine package from the Beijing Institute of Biology of China showed: “Generally, within 24 hours after vaccination, injection ministries may experience pain, tenderness, redness and itching, and in most cases they go away in 2-3 days. ” The other two Vaccine is marked as “see instructions for details”.
However, Kexing staff at the site told reporters that current clinical data shows that the main adverse reactions observed are local pain at the injection site, followed by fatigue, local swelling, etc., all of which are transient and only a few fevers are observed. And allergic reactions, no serious adverse reactions, indicating that the vaccine is safe.

Yin Weidong, President and CEO of Kexing Holding Biotechnology Co., Ltd.
Chinese biological field staff also mentioned to reporters that she herself had been vaccinated with the new corona vaccine developed by the company. “Before the clinical trial, some employees of our company took the initiative to vaccinate. The needle is very fine and I did not respond after the vaccine,” he said.
Relevant Kexing staff told reporters that he himself has been injected with the new corona vaccine developed by the company. “About 3,000 employees and family members of the company have been vaccinated against the new crown,” he said.
The reporter noted that a QR code called “New Crown Vaccination Appointment” was posted at the China Biotech booth. Staff told reporters: “We use this small program to understand the public’s willingness to vaccinate. By scanning the QR code, you can Participate in our COVID-19 vaccination intent survey. After recording the information, we will send you relevant information as soon as the vaccine is released. Currently, Chinese students studying abroad are more willing to get vaccinated. “
Research on the will of a new corona vaccination in the biological scenario of China
Staff from China Biology and Kexing told reporters that they expect the new corona vaccine to be listed by the end of 2020, and that the annual production capacity of both companies is also 300 million. China Biotech stated that the price will be a “public product”, a classified price and the principle of being accessible and affordable.Common peopleAffordable, use with confidence.

Massive information, accurate interpretation, all in the Sina Finance APP
Editor in Charge: Yin Yue