[ad_1]

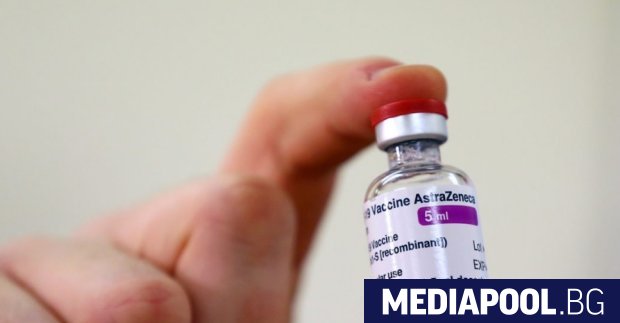
The Covid-19 vaccine from AstraZeneca and the University of Oxford has a new name, it is clear from the updated product information on the website of the European Medicines Agency (EMA). The new trade name for the vaccine is Vaxzevria, and so far it has been registered as Covid-19 Vaccine AstraZeneca. From now on both names will be used.
The last update of the product information was on March 26 and the name change request was approved by the European Agency on March 25. Product information has also been updated to include the risk of thromboembolic events. Updated product information is uploaded to the Medicines Executive Agency (BDA) website and you can familiarize yourself with it HERE.
The risk
New information on vaccine products warns that Very rarely, thrombosis in combination with thrombocytopenia has been observed after vaccination with Vaxzevria., in some cases accompanied by bleeding.
This includes severe cases of venous thrombosis, including abnormal localization, such as cerebral venous sinus thrombosis, mesenteric venous thrombosis, and arterial thrombosis accompanied by thrombocytopenia. Most of these cases occurred within the first seven to fourteen days after vaccination, in women under 55 years of age.
However, this may be due to the fact that this vaccine is given more frequently in this population. Some cases are fatal.
Healthcare professionals should watch out for signs and symptoms of thromboembolism and / or thrombocytopenia. Vaccinated persons should be instructed Seek immediate medical attention if symptoms such as shortness of breath, chest pain, leg swelling, persistent abdominal pain appear after vaccination.. In addition, anyone who has developed neurological symptoms after vaccination, including severe or persistent headache or blurred vision, or who has suffered skin bleeding (petechiae) after a few days away from the injection site, should seek medical attention. immediately.
The new trade name
Regarding the new commercial name of the vaccine, the Swedish Medicines Agency explained that the name change is allowed and will not lead to a change in the composition or action of the vaccine. However, you can to change the labeling and appearance. From there they specify that the purpose of the new trade name is to make the product stand out.
The Pfizer / BioNTech vaccine, for example, has the trade name Comirnaty, and before finalizing it, the manufacturer was discussing other options such as Rnaxcovi, Kovmerna and Covuity.
The trade name change for the AstraZeneca vaccine follows a series of reputational blows following its authorization for use in the EU. Initially, several countries restricted its use to adults older than 55 and 65 years due to a lack of data on its effectiveness in this age group, as the data available from clinical trials covered a small number of participants in this age group. . Just as the parties began to reconsider their position and allow it to older people, its use was discontinued for fear of an increased risk of blood clots (thrombi).
Although the European Medicines Agency (EMA) has concluded that the vaccine is safe, effective and far outweighs the potential risks, some countries remain secretive and concerns are compounded after each new reported thromboembolic event.

Did you find this article helpful?
We would be delighted to have you support the Mediapool.bg electronic edition, so that you can continue to have an independent, professional and honest means of analysis of information.
Support us
Subscribe to the most important news, analysis and commentary on the day’s events. The newsletter is sent to your email address every day at 18:00.
Subscription
[ad_2]