[ad_1]
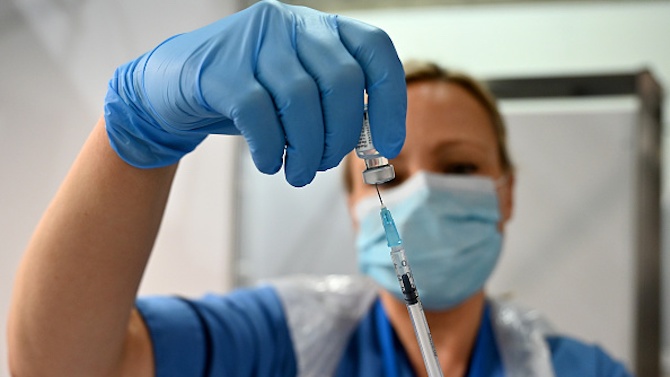
The European Commission today granted prior authorization for access to the EU market for the Pfizer vaccine against COVID-19.
All on the topic:
Coronavirus epidemic (COVID-19) 17157
Our decision is based on scientific evidence of the safety and efficacy of the vaccine, said EC President Ursula von der Leyen. Ursula von der Layen – German physician and politician (after Albrecht’s father). Ursula Gertrude Albrecht is. He noted that the approval of the Moderna vaccine is expected on January 6 next year.
Ursula von der Layen – German physician and politician (after Albrecht’s father). Ursula Gertrude Albrecht is. He noted that the approval of the Moderna vaccine is expected on January 6 next year.
Von der Layen pointed out that the first EU-approved vaccine against the new disease was the result of European success due to the work of the Biontech laboratories in the development of the vaccine. He added that researchers have received € 9 million from the EU in recent years to develop vaccine technology, and in June this year they received a € 100 million loan from the European Investment Bank. The president of the European Commission clarified that the first shipments of the approved vaccine come from production plants in Belgium.
They are expected to reach all European capitals, so immunization will begin on December 27 and 29.
By September next year, the EC expects to receive up to 300 million doses of vaccine in the EU. The Commission says that the first amounts will not be enough to immunize everyone, but adult Europeans may be covered by immunization until the end of next year. The EC adds that it is focusing on developing additional capacity for vaccine production. It is added that most EU countries provide free access to vaccines.
The leaflets on the effects of vaccines will be translated into all EU languages and will be available on the internet so that citizens can familiarize themselves with them in advance and do not request information from healthcare professionals. The EU makes an exception, since in the case of the first vaccines, it will not oblige manufacturers to supply packs with brochures in a language other than English, or to affix labels in the language of the country where deliveries are received .
The vaccines are intended to have a reference, such as a barcode, to a site with more information about the vaccine. The Commission notes that, together with the World Health Organization, it is developing vaccination passports (certificates) to care for those immunized on travel and prevent them from being subjected to testing and quarantine.
The EC clarifies that so far there is no data on whether those who have suffered from Kovid-19 need a vaccine. It is clarified that the preliminary market access permit issued today is related to the fact that the need to preserve public health outweighs the risk of side effects of the action of the new preparation.
All on the topic:
Coronavirus epidemic (COVID-19)
17157
More about the coronavirus

[ad_2]

