However Trump has predicted optimism for a quick timeline.
“[It’s] “It will be done in a very short time – maybe even during the month of October,” the president said in a press briefing on Monday. “We will have this vaccine soon, maybe before a particular date. You know what date I’m talking about.”
On August 6, Trump said he was “optimistic” that the vaccine would be ready by November 3.
“I think we’ll have the vaccine before the end of the year, for sure, but around that date, yes. I think so,” Trump said.
And he said at a rally last week that “it will be delivered before the end of the year, before the end of the year, but it will actually be delivered before the end of October.”
The federal official is not the first to throw skepticism at Trump’s prediction.
Dr. Larry Corey, who is leading a group set up by national health organizations to work on coronavirus vaccines, also said he did not think any vaccines would be available on election day.
Why don’t we have a vaccine till election day
In a word, hence probably. Vaccine will not be available before election day: Biology
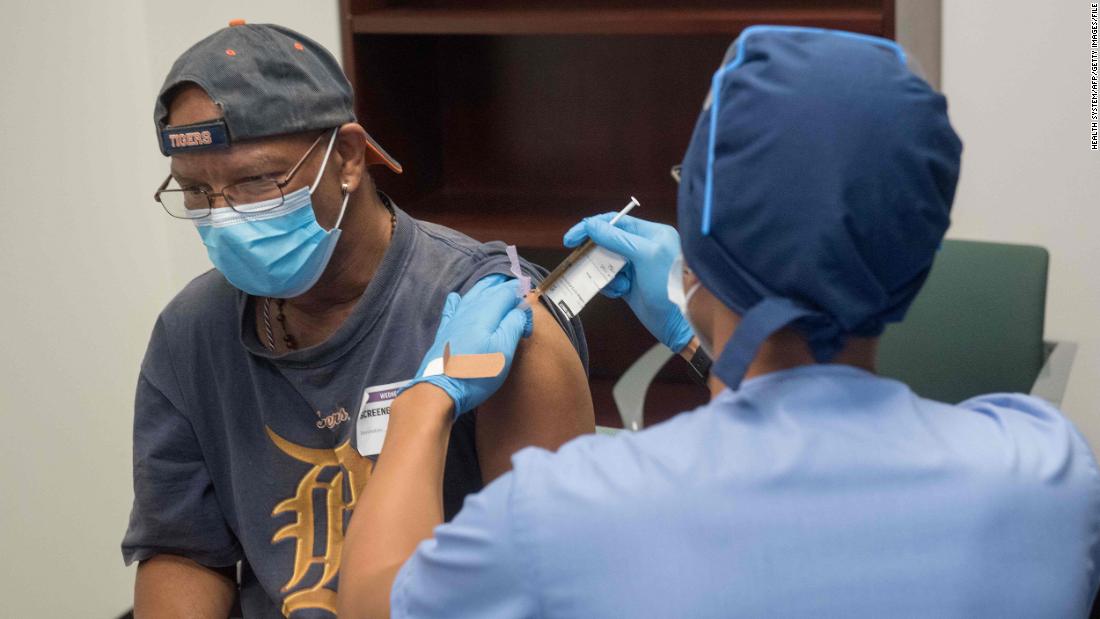
Here’s how the trial works: You take 30,000 people, give half of them a vaccine and give half of them a placebo, which is a salty shot that does nothing. Then those 30,000 people move on about their lives, and you wait to see how many people in each group get infected and get sick from the “endpoint”, Kovid-19, in the medical parlor.
It takes time to wait, especially since the current U.S. The coronavirus vaccine studied in is a two-dose vaccine with each dose except each week.
Vaccine manufacturers try to target locations and populations that deliver them quickly to their final point, but that doesn’t always work.
Dr. Robert Frank, director of the Vaccine Research Center at Cincinnati Children’s Hospital, wrote in an email to CNN that “everything depends on how quickly cases occur and then the number of cases in each group.” “It’s really the number one game.”
The length of that wait depends on how trial participants come into contact with the virus in their daily lives. Does the trial work outdoors among the people and religiously wear a mask when they come out in public? If so, it can take a long time for a significant number of infections to appear.
Cor historical illustrations as well as demographic information of people taking part in current coronavirus vaccine testing indicate the type of home stay.
It is not good to bring the trial to a quick conclusion.
Why are white-collar college-educated women bad for coronavirus vaccine trials?
Typically, people who volunteer for clinical trials tend to be “white, college-educated women” who have been chief investigators on dozens of vaccine clinical trials and have served on the Data and Safety Monitoring Board for many. Is.
All three of these factors are potentially bad news for coronavirus clinical trials, as data suggest that white college-educated women have a lower risk of exposure to the novel coronavirus.
Those factors help explain why whites are less likely to contract Covid-19.
When they make up 0% of the U.S. population, the U.S. for disease control and prevention. According to the Centers, whites account for only 1% of all U.S. cases.
Moderna and Pfizer have not published the education status of their participants, but if it is like the other tests, the volunteers are more likely to be college-educated.
That’s also bad news.
All of those factors help them stay away from coronaviruses.
Like women – that’s the third potential bad sign for the trial.
Not only are women more likely to be able to work from home, but they are also more likely to wear masks in public all the time.
Clinical trials often do not run as fast as researchers would like. But due to the urgency the Covid-19 vaccine tests are unusual. Researchers need trials to get Covid-19 cases on time so that the vaccine can enter the market and life can return to normal.
Covey, who runs the Covid-19 Prevention Network, noted that Pfizer and Moderna were the first two coronavirus vaccine tests to be launched, both of which were dosing their first volunteers on July 27. Downfall.
“This is the first test, and we don’t know how efficiently we will be able to achieve the determined endpoints of the trials. Will it be a straight, easily defined line, or will it be the same with the right step with a switchb with x? We’re in helpless water,” Corey said.
Another infectious disease specialist said researchers should be concerned that the endpoints will come slowly, especially those who join the tests.
Infectious Disease Specialist at Vanderbilt University Medical Center, Dr. “I can’t even imagine anyone worrying that the final points will be long in coming just for that reason,” said William Schaefner, an infectious disease specialist at Vanderbilt University Medical Center.
He personally knows college college-educated white women who have volunteered for Covid-19 vaccine tests: his daughter-in-law and his granddaughter.
“They wear masks. They make social distance. They’re careful.” “The next day, they came to our pool and they stayed at one end of the pool and we stayed on the other side. They never went inside the house. My wife told me, ‘Well, they’re not going to be helpful in the tests, that’s for sure.’
.