One of those mysteries: why can the experience be so very different from person to person. One expert says the answer may mean looking more closely at previous faxes that individuals have had.
“When we looked at the setting of Covid’s disease, we found that people who had previous vaccinations with a variety of vaccines – for pneumococcus, flu, hepatitis and others – appeared to have a lower risk of Covid’s disease. , “said Dr. Andrew Badley, an infectious disease specialist at the Mayo Clinic, told CNerson’s Anderson Cooper Monday night.
It’s what immunologists call immune training: how your immune system creates an effective response to fight infections, Badley says.
“A good analogy is to think of your immune system as a muscle,” he said. “The more you exercise that muscle, the stronger it will be when you need it.”
But once you are infected, how much of the virus made it into your body can also affect what your experience is, another expert told CNN Monday.
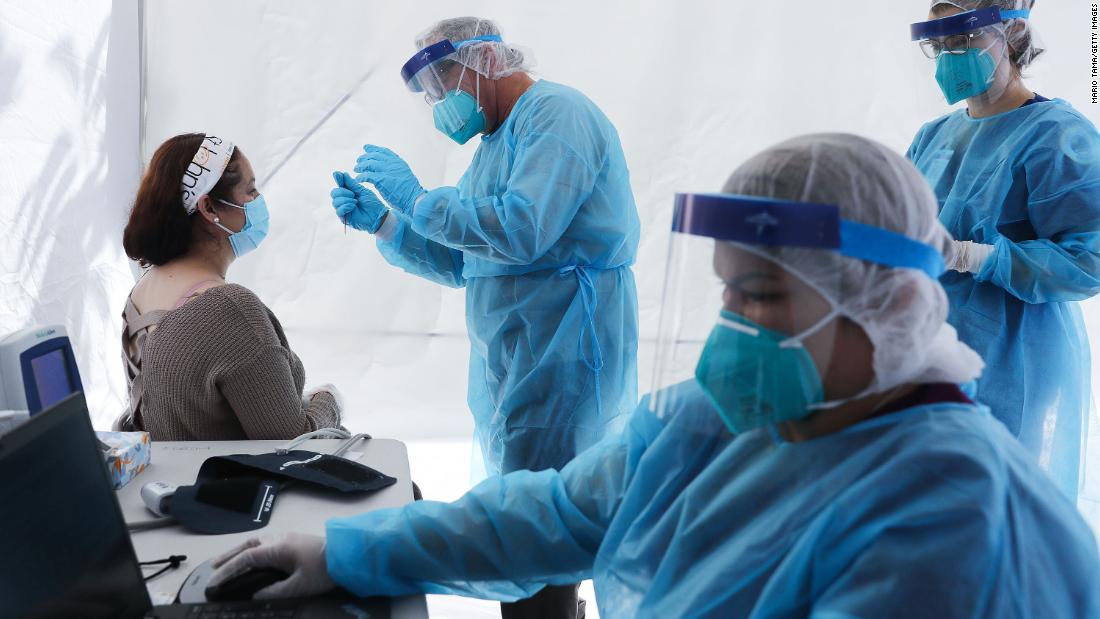
Gandhi’s team found that masks make a difference.
“What the mask does is really reduce the amount of virus you get in, if you get infected,” she said. “And by reducing that … you have a lower dose, you are able to manage it, you are able to have a calm reaction and you have mild symptoms if no symptoms at all.”
To date, more than five million Americans have tested positive for the virus and at least 163,461 have died.
90% increase in childbirths in one month
More research has also emerged on children and coronavirus in the middle of the entire school season.
According to a report published Monday by the American Academy of Pediatrics and the Association of Children’s Hospital, the number of coronavirus cases among American children has increased by 90% over four weeks.
The new report, expected to be updated weekly, said there were 179,990 new Covid-19 cases among U.S. children between July 9 and August 6.
More than 380,000 children were infected on August 6th, according to the report, accounts for well over 9% of total cases in states that report cases by age.
And although some U.S. officials, including the president, have said the virus does not pose a major risk to children, experts say that is not the case.
“It’s not fair to say that this virus is completely benign in children,” said Dr. Sean O’Leary, vice president of the American Academy of Pediatrics Committee on Infectious Diseases. “We’ve had 90 deaths in children in the U.S. in just a few months. Every year we raise our concerns about influenza in children, and there are about 100 deaths in children from influenza each year.”
In Mississippi, at least 22 schools across the state have reported positive cases, State Health Director Dr. Thomas Dobbs told a news conference this week. There were 19 cases reported among students and 15 cases among staff.
More volunteers added to trials of anti-therapy
Meanwhile, two clinical trials of anti-antibody therapy are registering more volunteers through the National Institute of Allergy and Infectious Disease Covid-19 Prevention Network (CoVPN), giving the studies a wider number of sites and a larger pool of people.
These therapies are antibodies made in the lab to target a particular infection like toxin. If they work, the treatments could immediately provide protection against the virus, compared to a vaccine, which can take a few weeks before it starts to protect and can not provide protection if one is already infected. But anti-body therapies usually last only a few months, while vaccines provide long-term protection.
In the first trial, volunteers were given a placebo as a dose of REGN-COV-2, an anticancer treatment made by Regeneron Pharmaceuticals, which has been shown to be successful in lab and animal studies and has been shown to be safe in human trials.
The trial will test to see if the cocktail of antibody provides protection against the disease and if anyone is infected, if the treatment limits the number of symptoms and if it helps keep people out of the hospital.
Scientists hope to enroll 2,000 asymptomatic adults who have had contact with someone in their household who has an infection. Volunteers would receive the treatment within a 96-hour window after they came in contact with the infected person and scientists would check with her again for seven months to make sure the treatment was safe and effective.
The second trial will test to see how Eli Lilly and Company’s LY-CoV555 antibody treatment works with people working or living in a nursing home or an assisted living facility who had a positive case.
That trial, looking for 2,400 volunteers, will test to see if the therapy prevents infections in this vulnerable population and will also test if the therapy prevents symptoms or reduces the severity of the disease.
CNN’s Jen Christensen and Jamiel Lynch contributed to this report.
.