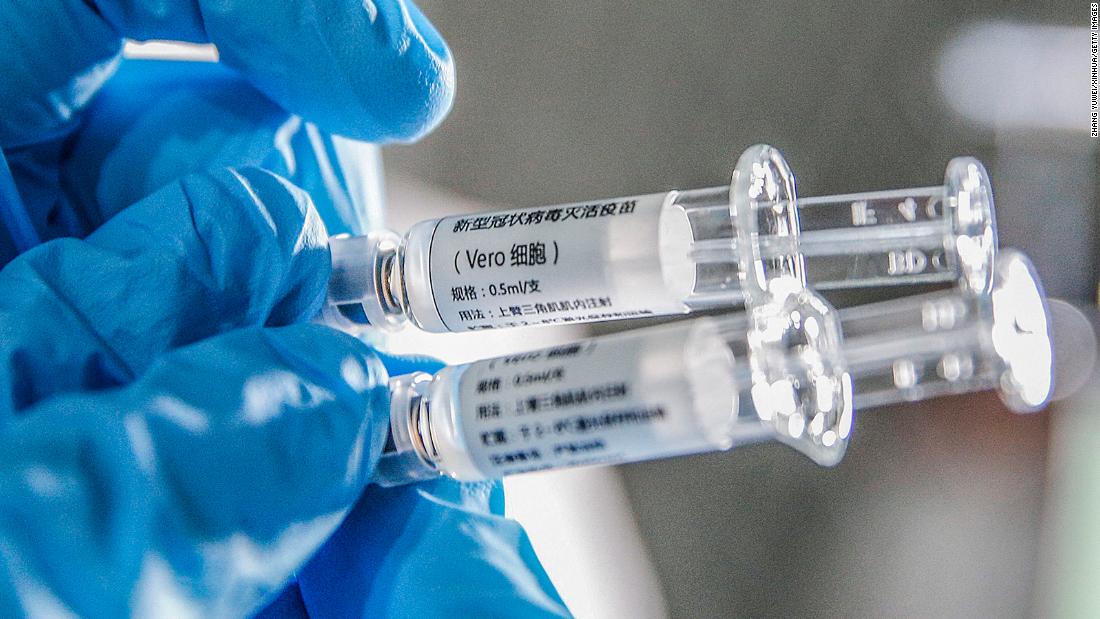
“In emergency use, we have now used it on about one million people. We have not received any reports of serious adverse reactions, and only a few people have some mild symptoms,” Liu said.
Liu said the vaccine was given to Chinese construction workers, diplomats and students who have traveled to more than 150 countries during the epidemic – and none of them have been infected.
He said in November that there were 1,000 emergency people who received emergency vaccines and then went abroad.
“For example, an international company has 99 employees in its overseas office fees, out of which vacc1 was vaccinated. And later, an outbreak in office fees, 10 out of 18 people not vaccinated became infected and none of them were vaccinated. , ”He said.
Separately, Sinofarm conducted Phase 3 clinical trials involving about 60,000 people from 10 countries, including the UAE, Bahrain, Egypt, Jordan, Peru and Argentina, he said. CNN has not yet seen the results of these tests.
Sinofarm has two vaccine candidates. It is unclear which vaccine Lu referred to
Since July, Chinese drug manufacturers have been offering experimental vaccines to people working in “high-risk” occupations under the Emergency Use program, allowing vaccine candidates to use them on a limited basis before fully proving their safety and effectiveness through clinical trials. .
Pfizer on Friday traveled to the U.S. for his vaccine. The Food and Drug Administration is expected to approve the emergency use.
Dr. Anthony Fauci, a top infectious disease specialist in the U.S., said the Pfizer and Moder vaccines have shown exceptional effectiveness.
“It’s extraordinary,” said phausie. “It’s almost at the level of what we want from measles, which is %%% effective.”
CNN’s Beijing bureau contributed to this article.
.