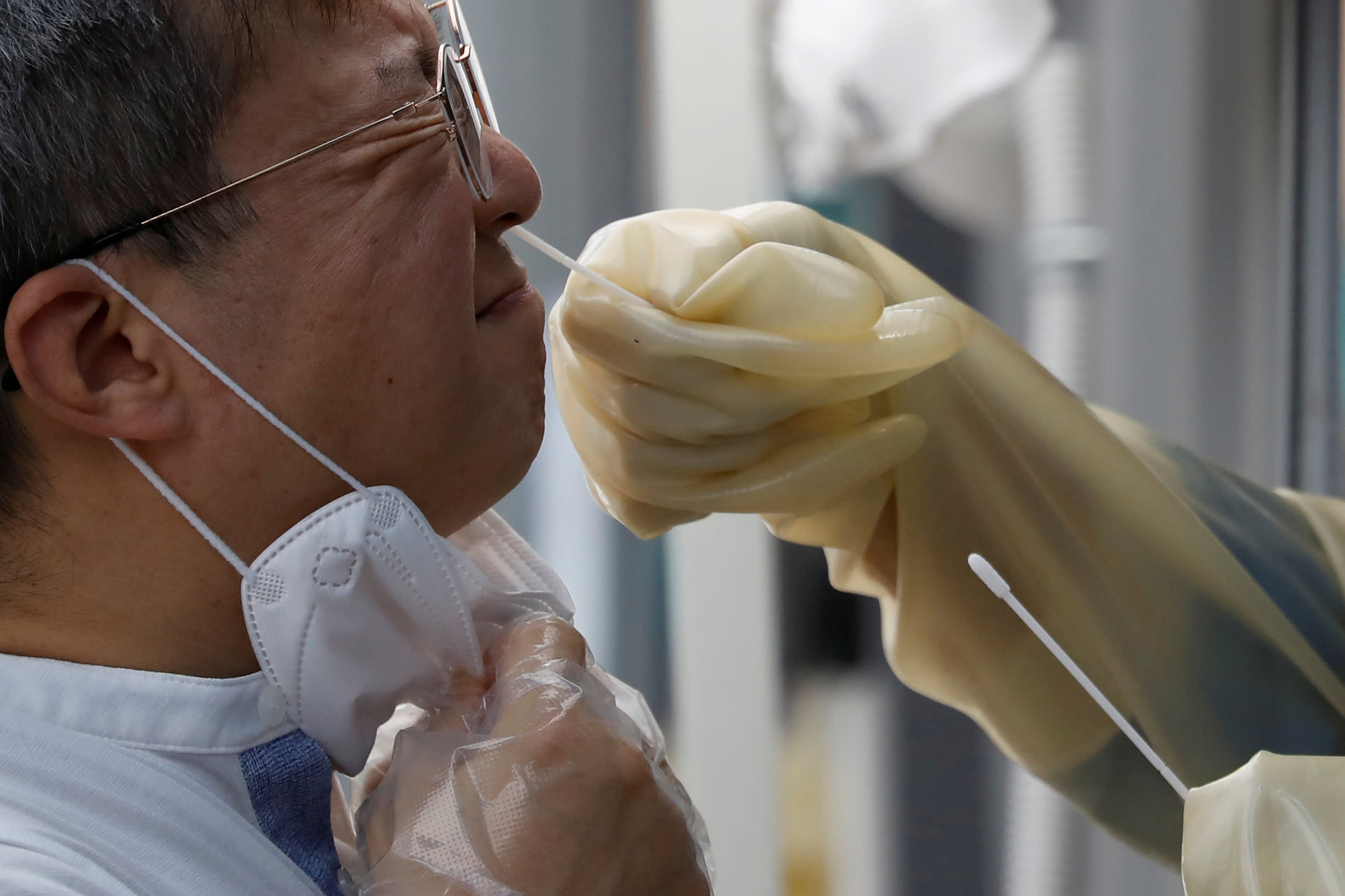
The Trump administration announced a $ 760 million deal with Abbott Laboratories (ABT) for a new rapid COVID-19 antigen test, giving new impetus to pressure to reopen schools and workplaces.
Testing for COVID-19 remains a bumpy ride in the U.S., with delays still occasionally reported in U.S. pockets, despite regular authorizations for emergency use by the U.S. Food and Drug Administration (FDA).
Over the past 24 hours, the FDA has authorized two new tests – a Fluidigm (FLDM) saliva test in California and Abbott’s $ 5 rapid antigen test. The latter is referred to entirely as game changer because it delivers accurate and fast results, and also includes an app that will host shared results. On Thursday, the U.S. Department of Health and Human Services (HHS) and Department of Defense (DoD) said they will buy 150 million tests.
The technology used is similar to home pregnancy tests, according to Abbott Division Vice President of Applied Research and Technology, Dr. John Hackett – except that it still requires a clinician to administer the test.
But a major reason it encourages enthusiasm is that it is the “only antigen test in the U.S. that does not require instrumentation,” Hackett said.
Almost all other tests require some sort of heavy-duty portable machine, including Abbott’s own IDNow rapid PCR test. The new test, BinaxNOW, is a handheld device and contains the necessary reagents to perform the test, which only takes 15 minutes to deliver results.
The administration has previously purchased other Abbott tests as part of an effort to clean up availability parts of the country, as well as add to inventory in the national stock. The company is starting production to send tens of millions of antigen tests in September and to produce at least 50 million a month in October.
The news comes at a time when schools in certain regions are moving to hold personal lessons at least part-time, and companies are trying to lure employees out of their work-from-home mode. Business Insider reported Thursday that Wall Street giant Blackstone intends for most of its workers to return to the office after Labor Day, and plans to send them COVID-19 tests at home to facilitate the transition.
Abbott is targeting schools and workplaces with the new test, saying the “health pass” for negative tests – which is valid for seven days – could help move people through lines faster to enter buildings. The antigen test is generally seen as accurate for positive results, but negative results may sometimes require a different type of test to confirm.
The speed and ease of the test can help to stimulate access to test, which is still tense at controls.
Recently, to satisfy peaks in some areas, large labs took steps to limit testing to a built-in large backlog – repeating the struggles the companies faced at the start of the pandemic. Although it only lasted a week or two, instead of months, the movement points to the ongoing struggle in test capacity.

The Dakotas see a parade in cases. (Graph: David Foster / Yahoo Finance)
Separately, the Centers for Disease Control and Prevention (CDC) have been relocated to clarify recently announced changes to test guidelines. In a controversial move, the bureau highlighted the need to test asymptomatic individuals, who blew criticism as a political maneuver to conceal positive cases – something President Donald Trump has previously indicated.
In a media call Wednesday, member of the White House Coronavirus Task Force Admiral Brett Giroir said the move was not intended to stop asymptomatic people altogether, but rather only in entire zones, if necessary.
However, the Infectious Disease Society of America said in a statement that the move was “concerning” and called for a reversal if cases continue worldwide, with symptom-less spread from a major driver. The pandemic has now affected more than 24 million, and killed more than 827,000. In the U.S., 5.8 million cases were exceeded and more than 180,000 Americans died.
Faxes on the horizon
As hope builds on a successful vaccine, Moderna (MRNA) and Pfizer (PFE) both presented data from their clinical trials to an advisory group at the CDC on Wednesday.
New insights into Moderna’s data showed that older adults responded to the vaccine in the same way as the younger population. Those older than 65, and in some cases as old as their 90s, produced similar neutralizing antibody responses, according to Moderna.
In a subsequent media call, executives said based on the positive news that the vaccine is likely to beat the FDA bar of 50%, possibly reaching 60% effectiveness.
A new center for infectious diseases
The National Institute of Allergy and Infectious Diseases has created a new center to study emerging infectious diseases that will include collaborations with 28 other countries.
The new channel of research reflects a collaborative effort traditionally seen at the World Health Organization. Healthcare providers are encouraged by the NIAID leadership, in the hope that the data will be less politically influenced
In a statement Thursday, NIAID announced 11 first-year grants totaling $ 17 million for the domestic and global research institutes that will form the Centers for Research in Emerging Infectious Diseases (CREID), with a commitment of at least $ 82 million over five years .
One of those lead researchers, Nikos Vasilakis, a professor and vice president of research at the University of Texas’ Medical Branch, told Yahoo Finance that the new collaboration of centers “brings the full strength, reputation and expertise” of the National Institutes of Health will contribute, as the funder, while current practices will be enhanced by the collaborative effort.
The new partnership is based on equality, inclusion and collaborations in the true sense of the word, Vasilakis said.
In the past, organizations have acted independently, with some researchers “helicoptering” to take samples, examine them, and then return to home settings – without credit to those who work on the ground in infected zones. That will all change now, Vasilakis said.
“This new program is a great start and a new basis for international cooperation and collaboration in this real threat we face. Especially now … we will start many more emerging events of nasty viruses affecting the world, Vasilakis said.
And the leadership of the new effort is a familiar sight, thanks to the current pandemic.
That is NIAID Director Anthony Fauci, who said in a statement. “The CREID network will enable early warnings of emerging diseases where they occur, which will be critical for rapid responses. The knowledge gained through this research will increase our readiness for future outbreaks,”
Several centers will focus on different regions of the world, including Central and South America, Asia and the Middle East. A coordinating center has been created in collaboration with RTI International in North Carolina’s Research Triangle Park.
@AnjKhem“data-reactid =” 65 “>Anjalee Khemlani is in reporter by Yahoo Finance. Follow her on Twitter: @AnjKhem
More from Anjalee:
Twitter,Facebook,Instagram,Flipboard,SmartNews,LinkedIn,YouTube.“data-reactid =” 72 “>Follow Yahoo Finance on Twitter, Facebook, Instagram, Flipboard, SmartNews, LinkedIn, YouTube.