MEDFORD, Erts. From the outside, it turns out to be just another suburban allergy clinic, a clean, tan brick-and-cinder block building set back from a busy highway and across the road from an auto parts store.
But inside the offices of the Clinical Research Institute in Southern Oregon, Dr. Edward Kerwin and his staff part of the race to save the world.
Kerwin, 63, was tapped this spring to lead one of nearly 90 U.S. clinical trials participating in the large-scale, phase 3 test of a vaccine produced by biotech startup Moderna to combat the phytus that COVID-19 causes.
In early July, Kerwin’s clinic, in a working-class region about halfway between Seattle and San Francisco, began registering up to 40 participants a day for the two-year study. He hopes to recruit as many as 700 volunteers by August.
They will participate in the 30,000 test subjects needed nationwide to determine whether the Moderna vaccine can tame a disease that has infected 5.4 million Americans and claimed the lives of more than 170,000. Another vaccine, produced by Pfizer and BioNTech, a German company, is being tested in nearly 30,000 more recruits.
“It’s a perfect opportunity for science to save,” said Kerwin, a tall figure in a bright blue shirt and khaki pants. He led visitors to a conference room, took a chair outside the socially distant reach and deafened his mask, how better to explain the scope of this moment.
Dr. Edward Kerwin, medical director of the Institute for Clinical Research in Southern Oregon, has conducted more than 750 clinical trials in the past quarter century. Kerwin, an allergist and immunologist, was applied as the lead researcher for Moderna’s COVID-19 vaccine study on the Medford test site.
He acknowledged “it may seem like a surprise” that Medford is the site of a clinical trial to stop the world’s biggest medical challenge in a century. But Kerwin, who worked as a NASA scientist before attending medical school and pursuing a career in allergy, asthma and immunology, has conducted more than 750 clinical trials in the past four years, mostly focusing on asthma, lung disease and skin disorders. .
He moved to southern Oregon in 1993, choosing the rural Rogue Valley because of its beauty and cultural opportunities, such as the Oregon Shakespeare Festival in Ashland. As his medical expertise grew, he built a top-notch clinical trial site that co-existed with a clinic that treats asthma and allergy patients. Along the way, he established deep roots in the valley, where he founded Bel Fiore, a $ 10 million winery and winery that owns a 19,000-square-foot chateau.
However, even with his experience, testing a vaccine to stop a global pandemic is a challenge like no other, Kerwin said. When the call came from Velocity Clinical Research – the North Carolina company that serves Kerwin’s clinic, known as CRISOR, and more than a dozen other COVID trial sites in the US – he paused for a moment.
“You take a big shock and say, ‘Do we have the resources to do this?'” Kerwin said. “You do it perfectly, but you want to do your homework.”
So far, testing is going well, he said. Unlike most clinical trials, for which it is difficult to recruit enough volunteers, the COVID effort has attracted intense interest. All Velocity sites paid participants $ 1,962 for the two-year trial, but Kerwin’s staff of two dozen initially did not advertise broadly.
“We would be worried that our phone would ring off the hook,” Kerwin said.
The Medford effort provides an example of the gamble drugmakers and federal trial sponsors take in deciding where to host large-scale clinical trials of COVID. To measure whether the vaccine works, you need to know that there is a good chance that participants will be exposed to the virus in the environment. Ethically, in traditional phase 3 trials, you can not intentionally infect humans with COVID, a disease without treatment or cure, although some suggest doing just that in controversial trials with human challenge.

Boxes containing bottles of Moderna’s COVID-19 vaccine, known as mRNA-1273, are refrigerated at the Clinical Research Institute of Southern Oregon in Medford.(Jim Craven for KHN)

Audrey Kuehl, a study coordinator at the Clinical Research Institute of Southern Oregon, keeps a dose of the vaccine used for the trial.(Jim Craven for KHN)
Southern Oregon has not been a hot spot for COVID, with fewer than 500 confirmed cases and two deaths in Jackson County, which includes Medford. But, Kerwin said, it’s the risk of becoming one, it offers the opportunity to vaccinate subjects before the virus spreads.
“It’s almost too late in New York and Arizona,” he said.
In the meantime, he is trying to shift the chance that trial volunteers will be exposed to COVID-19 by reaching out to people at greater risk for infection.
That Kerwin’s team has sought contact with companies in industries such as agriculture and food production, where it is known that the disease has spread with certain virulence. Locally, that includes employers like Harry & David, the food retailer famous for its fruit-of-the-month shipments, and Amy’s Kitchen, the maker of vegetarian frozen meals, which operates a production plant in the area.
The Medford trial site also highlights enrollment of older volunteers, those aged 65 and over, who are at higher risk for serious illness than coronavirus death.
One of the first volunteers was Trish Malone, a 68-year-old cultural anthropologist living in Ashland. Like many of the other participants, she has enrolled in Kerwin’s previous clinical trials of asthma treatment devices. When clinic staff members came to ask if they participated in the COVID trial, they did not force it.
“I said, ‘Wow, yeah,'” Malone reminded her. ‘It’s because [Kerwin] and his expertise. Little Medford gets these tests. “
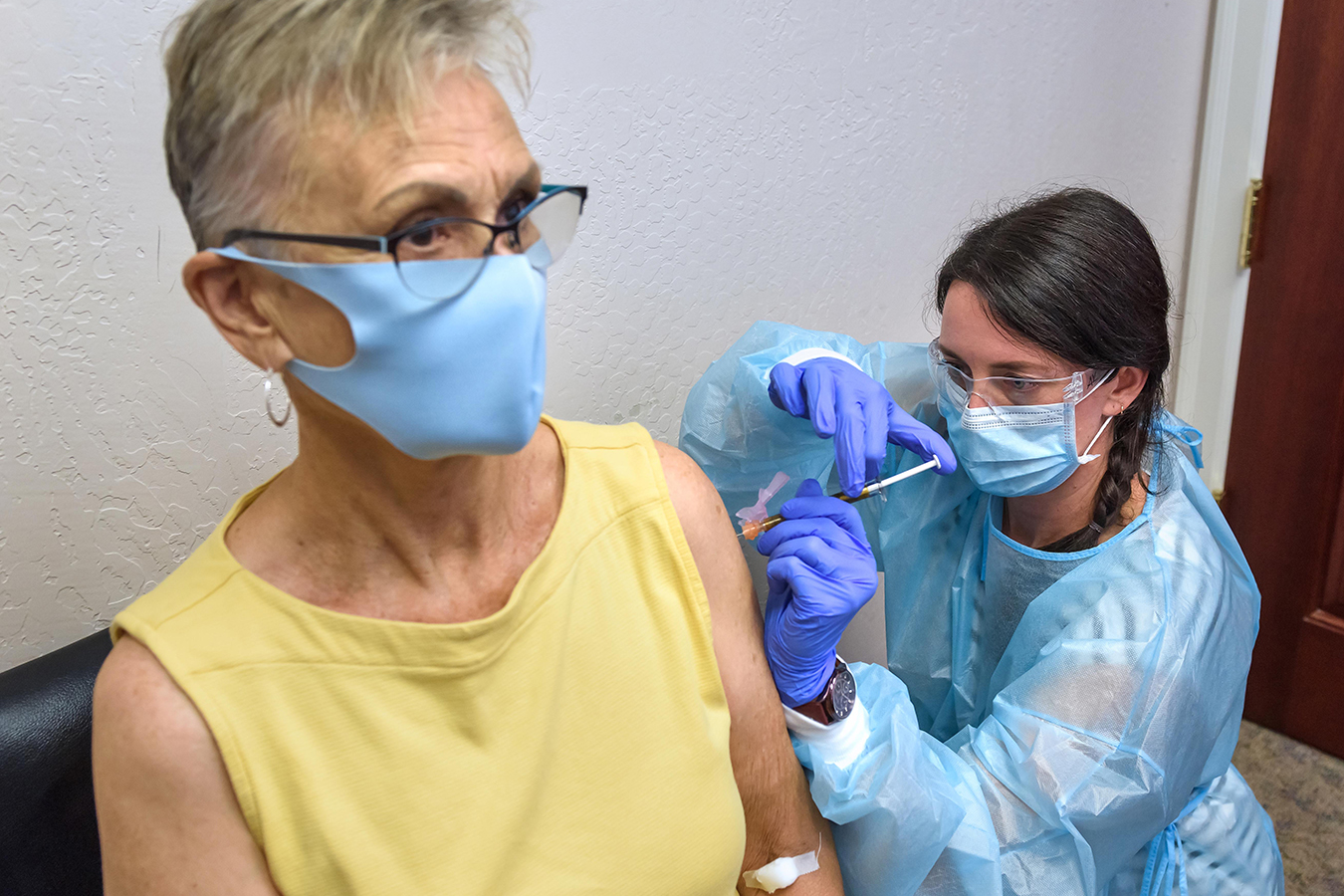
Participating is a way to ‘give back’ to her community, said Malone, who sat calmly and quietly on a recent Thursday when study coordinator Audrey Kuehl dropped the injection into Malone’s left shoulder.
Audrey Kuehl, a study coordinator at the Clinical Research Institute of Southern Oregon, inoculates Trish Malone with Moderna’s COVID-19 vaccine on August 6.
‘She was fast. It was not a pain, and it was fine, ”said Malone.
Half of the patients in the trial will receive two doses, 28 days apart, of the Moderna vaccine, called mRNA-1273. It uses a piece of the genetic code of the coronavirus, not the virus itself, to instruct cells to produce a protein that triggers an immune response to protect against infection. The other half will receive a placebo, as a saline dummy shot.
Three study coordinators at Medford Clinic, Kuehl among them, know which patients receive which dose, but the information is kept from volunteers and other staff members – including Kerwin, the lead researcher.
Participants receiving the vaccine may experience some side effects, such as injection site redness, muscle cramps, fatigue or headaches, Kerwin said. “It’s a sign that the vaccine is working with your immune system,” he said.
Four days after her first injection, Malone was disappointed not to report a reaction. “I’m bummed, totally bummed,” she said. ‘I have no symptoms. I think I got the placebo. ‘
That may not be true. Even if it is, Malone said, she is happy to take part in an effort that could help stop the deadly virus.
“This is a global pandemic,” she said. “What can I do to help?”
The study will run for two years, allowing researchers to track the long-term effects of the vaccine. Malone will keep a diary of her temperature and symptoms, if any, and have regular blood tests to determine if she has antibodies to the virus.
Kerwin is optimistic about the chances that the Moderna vaccine will work, agreements with Dr. Anthony Fauci, the expert from the summit of infectious disease, who predicted that the study could demonstrate effectiveness by November or December. Kerwin estimates that the vaccine could prove 90% effective, although experts outside of infectious disease said it was far too soon to tell.
Even if the trial shows that the vaccine is successful, it would take months longer to produce and deliver enough injections for the US and beyond.
As he enrolls patients and waits for data, Kerwin said, he is thinking about the real-world implications of his work. His mother, in her 90s, lives in a nursing home in Denver, where to date there have been no cases of COVID-19. But the threat loomed.
The tragedy of the pandemic has underlined the promise of science – and the interconnectedness of people far beyond this small corner of Oregon.
“Immunology has never been more fascinating than it is today,” he said. “This is a year that reminds us that we can not live in isolation and not live in isolation from the world.”