
[ad_1]
Tonight, 9-12, the Ministry of Health published a press release on the official approval and implementation of the first phase of the clinical trial of the Nanocovax vaccine from Nanogen Biotechnology Joint Stock Company (Nanoge Company). ) at the Academy of Military Medicine.
Accordingly, on December 9, the National Research Ethics Council held a meeting to evaluate and approve the research documents for the Phase 1-2 clinical trial of the Nanocovax vaccine produced by the Nanogen Company. and assigned the Military Medical Academy research group to lead this clinical trial research topic.
The National Council of Biomedical Ethics has approved a plan to recruit volunteers to participate in phase 1 research, it is expected that, tomorrow December 10, the Military Medical Academy will launch the clinical trials program. Covid-19 vaccine and announced the recruitment of volunteers to participate in the research; On December 17, the first vaccine will be delivered to volunteers participating in the phase 1 study.
Under the plan, on December 10, the Military Medical Academy officially announced the recruitment of volunteers to participate in the first phase clinical trial study of the Nanogen Company’s Nanocovax Covid-19 vaccine and began the trial program. vaccine testing in Vietnam.
The number, selection and exclusion criteria of the research participants and the content of the information provided to the research volunteers have been approved by the National Ethical Council in Biomedical Research.
Consequently, phase 1 will have 60 healthy volunteers between 18 and 50 years old who will participate and will be randomly assigned to 3 groups, including group 1a with 20 users with a dose of 25 mcg, who will be recruited first. followed by group 1b of 20 users taking a 50 mcg dose and then group 1c of 20 taking a 75 mcg dose. All subjects participating in the phase 1 study will receive 2 intramuscular injections of the vaccine, the interval between 2 injections is 28 days.
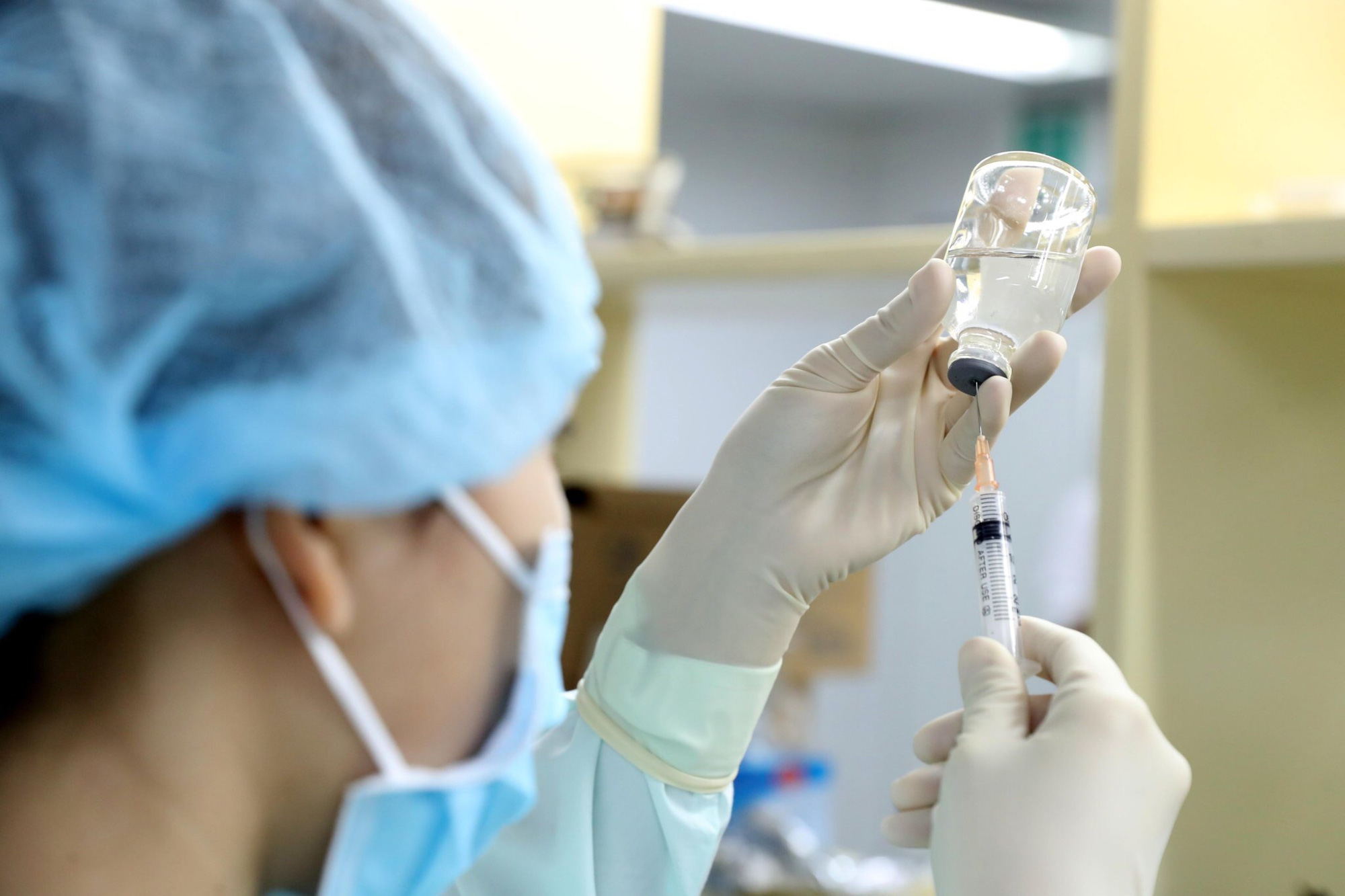
Research on the Covid-19 vaccine in Vietnam- Photo: Ngoc Thanh
To ensure the safety of study participants, during the recruitment period of 60 participants, the safety assessment was performed according to the escalating dose detection design. Selection of participants in phase 1 will begin with three people in the 25 mcg dose group. Based on the results of follow-up and evaluation after 72 hours after vaccination in these first 3 people, the dose level and number of participants will be decided next. The duration of the study per participant was approximately 56 days for the evaluation of the study objective and the follow-up until the sixth month from the first injection.
According to the plan, on December 17 it will deliver the first vaccine to volunteers who are eligible to participate in the phase 1 study.
Based on the research protocol approved by the National Ethics Council, the Ministry of Health will direct and organize the mission of monitoring and supervising the entire research implementation process of the research team to ensure that it is absolutely safe for the subjects that are volunteer to participate in research.
Before that, the Ministry of Health expected 40 people between the ages of 18 and 40 to participate in the phase 1 trial of Covid-19 with 40 people. Initially, the volunteers were divided into 2 groups with 2 dose ranges: 50 mcg and 75 mcg / nose. In particular, 20 people will be injected with a dose of 50 mcg / injection, then 20 people will be injected with the remaining dose to evaluate the optimal dose range before moving on to phase 2 (evaluation of the immunogenicity calculation ) with many age groups. Phase 2 is scheduled for March 2021, studying larger numbers (minimum 400 people) to further evaluate safety and immunogenicity.
[ad_2]