[ad_1]

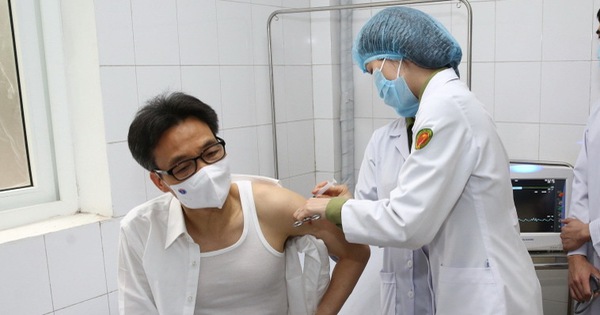
Vice Premier Vu Duc Dam has a COVID-19 Nano Covax trial vaccination – Photo: VOV
On the morning of March 26, at the Military Medical Academy (Ministry of Defense), Deputy Prime Minister Vu Duc Dam participated in the trial injection of the COVID-19 Nano Covax vaccine, according to VOV. This is a vaccine produced by Vietnam and is in phase 2 human trials.
Before vaccination, Vice Premier Vu Duc Dam was checked and examined and followed for 30 minutes in accordance with regulations.
Then, Mr. Dam asked and encouraged the volunteers to participate in the trial phase of the Nano Covax vaccine.
Mr. Dam thanked the volunteers who participated in the vaccine trial researched and developed by Vietnamese scientists.
According to him, each volunteer is directly contributing to the vaccine research and development process in Vietnam in order to obtain the COVID-19 vaccine for disease prevention in the shortest time possible.
This is also the pride and confidence in the Vietnamese vaccine research and development team of scientists, also the desire of the people.

Vice Premier Vu Duc Dam encouraged and thanked the volunteers who participated in the Nano Covax vaccine trial – Photo: VGP / Dinh Nam
A 68-year-old volunteer (in Dong Hung district, Thai Binh province) said that after the first injection on February 26, he did not notice any unusual reactions, such as injection pain or mild fever. For 7 consecutive days, he fully complied with the instructions on continuous home health monitoring.
The volunteers expressed their confidence in the success of the vaccines researched and developed by a team of Vietnamese scientists and hoped that in the near future, all people will be vaccinated against COVID-19 Manufactured in Vietnam.
Regarding the information on the Nano Covax vaccination trial, Lieutenant General, Prof. Dr. Do Quyet, director of the Military Medical Academy, said with the fierce participation of the Government, the Ministry of Defense, the Ministry of Health, that now is the time The research and development of the Nano Covax vaccine has been reduced to the maximum possible level , but it still guarantees the safety, scientific evaluation and accuracy of the immunogenicity of the vaccine, with the goal of having a safe and effective vaccine for the Vietnamese soon.
Phase 1 of the Nano Covax vaccine trial is very safe, produces good antibodies, has good ability to neutralize the virus and already has antibodies against the new UK strain of virus (B117).
Phase 2 tests vaccines with a larger number of samples than phase 1 (with 560 volunteers), continues to do double and multicenter trials to evaluate safety, pay more attention to efficacy, antibiotics, antibodies and their antiviral capacity. The planned end of April 2021 will finalize the second injection of phase 2.
“With current progress, at the end of June and beginning of July 2021, it is possible to present to the National Council of Ethics in Biomedical Research through phase 3, which can authorize the test for a wider area.”
Until now, the research and production time of the Nano Covax vaccine has been shortened to the maximum possible, but it still guarantees the science and safety to soon have a safe and effective vaccine for the Vietnamese, “said the director. Military Medical Academy said director.

Lieutenant General, Prof. Dr. Do Quyet – Director of the Military Medical Academy: If convenient, by September 2021 we will have the first COVID-19 vaccine in Vietnam – Photo: VGP / Dinh Nam
He also said that phase 3 tests will need about 10,000 people, including 5,000 injections. Vaccine AstraZeneca and 5000 injections Vaccine Nano Covax to have a scientific basis to compare side effects, unwanted effects, efficiency of antibody production and virus elimination. With such an approach, we can fully do it in Vietnamese conditions.
“I think if it is favorable, by September 2021 we will have the first COVID-19 vaccine in Vietnam, even this possibility is very high. We have the right to believe that it will be successful,” said Prof. Dr. Do Decision. .
Nano Covax is the first vaccine in Vietnam to be injected for human clinical trials, investigated by Nanogen Biotechnology Joint Stock Company. They participated in the phase 2 trial injected with 560 volunteers, extending the subjects from 18 to more than 60 years, in which some people had underlying diseases such as hypertension, dyslipidemia, diabetes, cardiology grade 1. … not too heavy.
The volunteers were divided into 4 test injection groups, of which 80 received placebo injections; The remaining volunteers were divided into three dose groups of 25 mcg, 50 mcg, and 75 mcg.
The test injection this time has 105 volunteers over 60 years old; volunteer over 76 years old. The trial injection of phase 2 of the COVID-19 Nano Covax vaccine was carried out at two locations: the Military Medical Academy and the Pasteur Institute of Ho Chi Minh City (participating in the study, conducted in the district of Ben Luc, Long An Province).