[ad_1]
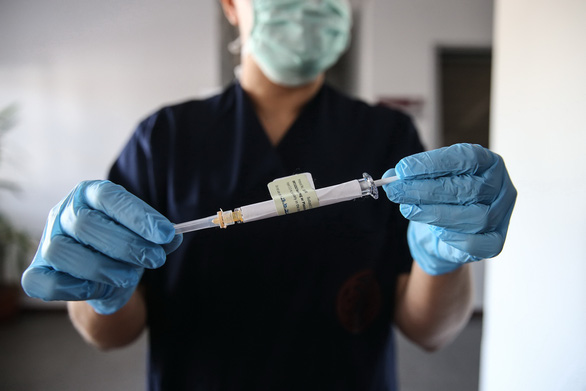
A healthcare worker in Turkey holding a syringe in a phase 3 clinical trial project with the COVID-19 vaccine co-developed by Pfizer and BioNTech – Photo: AA / Getty Images
This positive information immediately generated hope in the world community, as the global number of COVID-19 cases surpassed 51 million people and more than 1.2 million people died.
High efficiency
The Pfizer report that evaluated 94 cases of COVID-19 was identified among 43,538 study participants. Based on the initial evaluation, there have been no serious complications from the vaccine, but data on the safety and efficacy of the vaccine will continue to be collected.
A protective effect of more than 90% makes BNT162b2 expected to be equivalent to vaccines with a high protective efficacy such as the measles vaccine.
According to Pfizer, by the end of this year, Pfizer and BioNTech will have enough doses of the BNT162b2 vaccine to inject between 15 and 20 million people. By March 2021, the company could distribute 100 million doses of the BNT162b2 vaccine according to US orders, and it is expected to produce 1.3 billion doses by 2021.
However, that is not enough to meet the needs of some 7.8 billion people in the world. Pfizer will have to calculate the limited supply of the vaccine to the US (which may also get an additional 500 million doses) and pre-order countries like the UK, Japan and Canada.
The Food and Drug Administration (FDA) says it has an external vaccine advisory committee that will help evaluate the effectiveness of all COVID-19 vaccines and make it public.
Members of that committee will help provide an objective evaluation, complementing an important evaluation step with independent evaluation by FDA staff. It is that committee that ultimately decides which COVID-19 vaccine deserves FDA approval.
The FDA itself recommended that the developers of BNT162b2 not apply for emergency approval with this drug until after two months of vaccination of at least half of the volunteers.
I still have to wait
Although the vaccine development company claims that the effectiveness of BNT162b2 is 90%, the New Scientist site warns that it is very likely not that good.
First, the data analysis published by Pfizer and BioNTech in the press release is not conclusive information. It is not yet a research report published in peer-reviewed specialized medical journals.
According to Politico, in the coming weeks, public opinion will have more complete information on whether Pfizer’s vaccine is safe.
Pfizer’s Sept. 11 press release summarizes early data from the 44,000-person Phase 3 clinical trial study, but there is no safety data, including types. what side effects were found and how severe they were recorded in the trial participants. This information is likely to be more complete in Pfizer’s emergency license application with the FDA.
Only when Pfizer has approved BNT162b2 by the FDA will it be known whether the vaccine is effective for everyone because older adults tend to have weaker immune systems or children also have a response mechanism to the vaccine from other adults.
Pfizer is also testing its COVID-19 vaccine in people with an underlying medical condition such as HIV or hepatitis C. They actively screen volunteers of different races and ethnicities for the most multidimensional evaluation. on the effects of BNT162b2.
In their study, Pfizer verified the efficacy of the vaccine by comparing how many clinical trial participants became infected with the corona virus during a week after receiving a second dose of the BNT162b2 vaccine.
However, 7 days is a short window period, so scientists still want to know if the vaccine can be effective for longer.
More optimistic signs
In the current COVID-19 vaccine development race, in addition to the advancement of Pfizer, Moderna, AstraZeneca, and Johnson & Johnson also have potential vaccine candidates that are in the final stages of clinical trials. Another company, Novavax, also plans to launch trials in the second half of this month.
Moderna, which also has a vaccine that uses mRNA technology like Pfizer’s BNT162b2, is expected to release its safety assessment data in November. AstraZeneca may publish data this month or next. Johnson & Johnson will have scientific evidence of the safety of its vaccines this year.
Scientists and governments hope to have more than one effective COVID-19 vaccine. That will increase the number of vaccine doses given, increasing the likelihood that at least one will work for specific groups, such as the elderly, children, and pregnant women.
Vietnam vaccines also joined the race
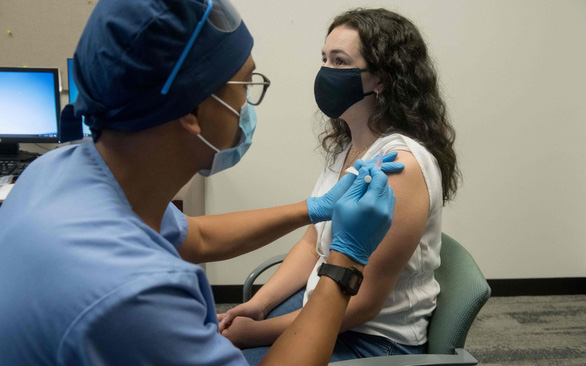
A volunteer participates in a clinical trial of the COVID-19 vaccine developed by Moderna – Photo: AFP
Four Vietnamese vaccine manufacturers are participating in the race to develop the COVID-19 vaccine. The first of these is the product of the Nanogen Company, which is currently in the stage of completing the documents for the phase 1 clinical trial.
During a meeting a week ago between the Ministry of Health, experts in vaccine testing and analysis, the Military Medical Academy, which will conduct clinical trials and vaccine development units, Nanogen Company announced the design of clinical trials.
Consequently, phase 1 (expected to be implemented in December) is a non-randomized clinical study, gradually increasing the dose, evaluating the safety, tolerance level and immunogenicity of the Nanocovax vaccine from Nanogen Company. The company designed this phase to test 60 healthy volunteers, but the Ministry of Health recommends testing 20 people first.
Phase 2, expected about 3 months after the phase 1 trial, will be a multicenter, double-blind, randomized clinical trial comparing placebo to assess safety, efficacy, and maximum dose. advantages of the Nanocovax vaccine. This phase is expected to be tested in 400 people, including those taking the vaccine, some not taking it, at many different testing centers.
The Health Ministry said the manufacturer’s clinical trial records are being reviewed before being submitted to the Ethics Council in biomedical research and official tests.
In addition, another vaccine manufacturer, the Nha Trang Medical Biologics and Vaccines Institute, is also in the final stage of preclinical evaluation, and this manufacturer also has the idea of designing a clinical trial. When there is enough data, the preclinical stage will be submitted to the Ministry of Health for approval of clinical trials in humans, which are expected to arrive in 2021.
Regarding the dose of the COVID-19 vaccine, there is currently no vaccine that has officially announced the optimal dose, nor is there any vaccine with information on the duration of protection after vaccination. However, according to one expert, only when 80% of the community is vaccinated will it have a broad protective effect.
Therefore, with the expected production capacity of the vaccine, it will take 2 to 3 years to broadly “cover” this vaccine. And Vietnamese vaccines have the opportunity to enter the market, when according to the forecasts of the Ministry of Health, if the tests go well, by the end of 2021, the Vietnamese vaccine will complete the 3 stages of human tests, standard registered for circulation in the market.
L.ANH – X.LONG
Mr. KIM THOUA
[ad_2]