[ad_1]
40 volunteers received a Covid-19 vaccine test on December 10. Nanogen is considering sales prices of no more than 500,000 VND per dose.
40 people will be evaluated first
Only 2 days left, the first batch of Covid-19 preventive vaccines in Vietnam by the Military Medical Academy and Nanogen Pharmaceutical Biotechnology Co., Ltd. will officially enter phase 1 testing in 40 people after 1 week. recruitment of volunteers.
After 6 months of research, based on recombinant protein production technology, preclinical studies in monkeys and mice, this vaccine has shown safe results and has an immune response.

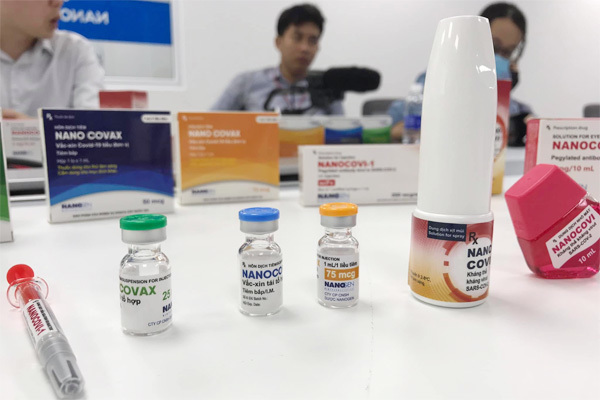
The first vials of the Covid-19 Nanocovax vaccine before the test time
Dr. Do Minh Si, Director of Research and Development at Nanogen Biotechnology Joint Stock Company, said: “Like any drug or vaccine, before being put to the test, Nanogen goes through one step. very important is preclinical research.
“This proves two things: first it is safe in animals, the second is the ability to induce an immune response to the nCoV virus. With Covid-19, everything is improved very quickly to keep up with the progress of clinical trials.”
According to Dr., before being tested, the company did research to create cell lines to produce antigens, then did research to create a finished vaccine, and finally coordinated with many different organs to test and evaluate vaccines.
However, the most difficult step is preclinical testing to demonstrate immunity in animals.
Dr. Do Minh Si also reported that 40 people, ages 18 to 40, will be vaccinated in phase 1. The test unit will administer a test injection to the first 1-2 people in the volunteer group, wait for a response and determine safety for the first 72 hours, then inject the rest of the people.
The volunteers who administered the Covid-19 vaccine trial were people who did not have an underlying disease. These individuals are carefully exploited for their health history, allergies such as medications, foods … because these local factors are strongly related to post-injection reactions. After the first trial phase, phase 2 is expected to inject 400 people over 4 months.
“There are expected to be around 2 million doses of vaccine in forms like injections, nasal sprays, and eye drops tested by the manufacturing company. If the pilot is successful, it will increase the capacity to 50 million vaccine doses per year, ”said Dr. Dr.

Medical staff distributing test samples
The vaccine may be covered by insurance.
According to Dr., the company considers the price of the Nanocovax vaccine, which is expected not to exceed 500,000 VND per dose.
Priced not to exceed VND 500,000, Dr. Doctor said, this is a reasonable level for everyone to use. In addition, the company is trying to include Nanocovax in the list of drugs covered by health insurance. The vaccine will require 2 injections for ages 12 to 75, 28 days apart, you will have immunity within 1 year and must be repeated.
Currently the company carries out research and production independently, the initial investment costs are borne by the company. The company only imports chemical kits to test whether the product is pure or not and how effective it is, the rest of its vaccine materials are self-produced.
“If the subclinical trial is favorable, it is expected that by the second quarter of 2021, the first Covid-19 vaccine in Vietnam will be widely used,” said Dr.
| Currently, in addition to Nanogen Company, Vietnam also has 3 units that produce, research and produce Covid-19 vaccines, including: Vaccine and Medical Biological Products Institute (IVAC), LLC a member of Vaccines and Biologicals. Product No. 1 (Vabiotech) and Research Center for the Manufacture of Vaccines and Medical Biological Products (Polyvac). After testing the Nanogen Company’s Covid-19 vaccine, scheduled for February 2021, the IVAC vaccine will be vaccinated and in March 2021 it will be Vabiotech vaccine. Vaccines from these units are also evaluated for safety and immunity in animals. |
Lien anh
Who can choose to try the Vietnam Covid-19 vaccine?
In phase 1, Nanogen will select 60 healthy volunteers. The clinical trial is expected to be completed in April.