[ad_1]
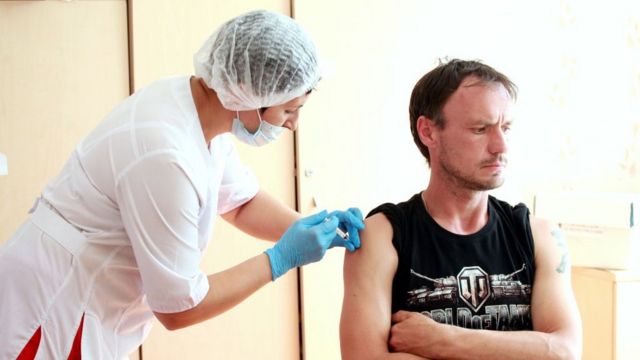
Image source, Kirill Shipitsin / Getty Images
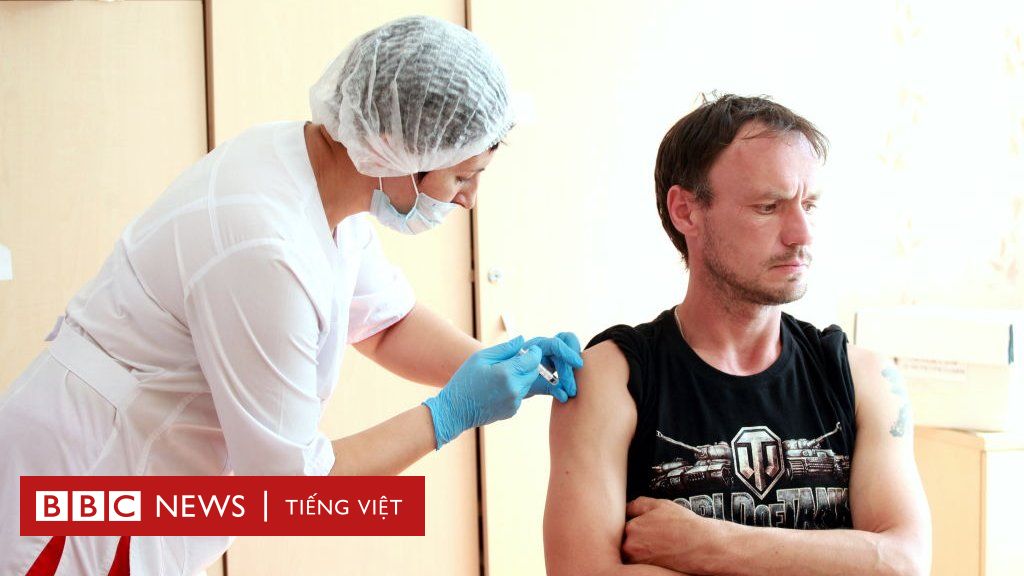
Russian scientists released the country’s first report on the Covid-19 vaccine, saying initial tests showed signs of an immune response.
The report, published by The Lancet medical journal, says that those who received the test injections developed antibodies to fight the virus and had no serious side effects.
Russia was the first country to license the Covid-19 vaccine in August, before the aforementioned data was released.
Experts say these tests are too small to be effective and safe.
But Moscow hailed the results as a response to critics. Some Western experts have raised concerns about Russia’s speed of work, saying that researchers may be shortening the process.
Last month, President Vladimir Putin said the vaccine had passed all mandatory tests and that his daughter had received the vaccine.
What does the report say?
The Lancet reported that two vaccine trials, called Sputnik-V, were conducted between June and July. 38 healthy volunteers received a dose of the vaccine and then a booster shot three weeks later.
The participants, between the ages of 18 and 60, were followed for 42 days and all developed antibodies within three weeks. The most common side effects are headache and joint pain.
The trials were public and non-randomized, meaning that there was no placebo and the volunteers knew they had been vaccinated.
“Large long-term trials including the placebo comparison and additional follow-up are needed to establish the long-term safety and efficacy of the Covid-19 vaccine,” the report said.
According to the article, the third phase of the trial will involve 40,000 volunteers of “different age groups and at risk.”
Russian vaccines use adapted strains of adenovirus, a virus that often causes the common cold, to trigger an immune response.
There is still a long way to go
Philippa Roxby, BBC Health Reporter
“Encouraging” and “so far very good” were some of the responses from UK scientists, but it is clear that there is still a long way to go. Although the vaccine showed antibody responses in all participants during phase two, this does not necessarily mean that it will protect them from the virus. That is not yet guaranteed.
From these results, we can say that the vaccine appears to be safe in healthy people aged 18 to 60 for 42 days, because that is when the research is in progress. But what about the elderly and those with an underlying medical condition at increased risk for Covid-19? How safe is it for them and for a longer period of time?
This can only be resolved after much larger long-term randomized trials in which participants did not know if they were getting a vaccine or if they had been given a sham injection. These will also tell scientists how effective the vaccine is in the wider community.
There have also been calls for openness and transparency. Of the many vaccines currently being tested around the world, some will work better than others in certain cases and perhaps in certain groups of people. Therefore, knowing exactly how well they work and who they are used for is paramount; one vaccine is unlikely to be right for everyone.
Reactions?
Kirill Dmitriev, director of a Russian investment fund and the man behind the vaccine project, told a news conference that the report was “a forceful response to skeptics who have been unreasonably criticized.” Management of Russian vaccines “.
He said 3,000 people were selected for the next testing phase.
Russian Health Minister Mikhail Murashko said the country will start vaccination from November or December, which focuses on high-risk groups.
However, experts warn that there is still a long way to go before a vaccine can reach the market.
According to the World Health Organization, 176 potential vaccines are currently being developed around the world. Of these, 34 are currently being tested in humans. Among them, eight vaccines are in stage three, the most advanced stage.