[ad_1]
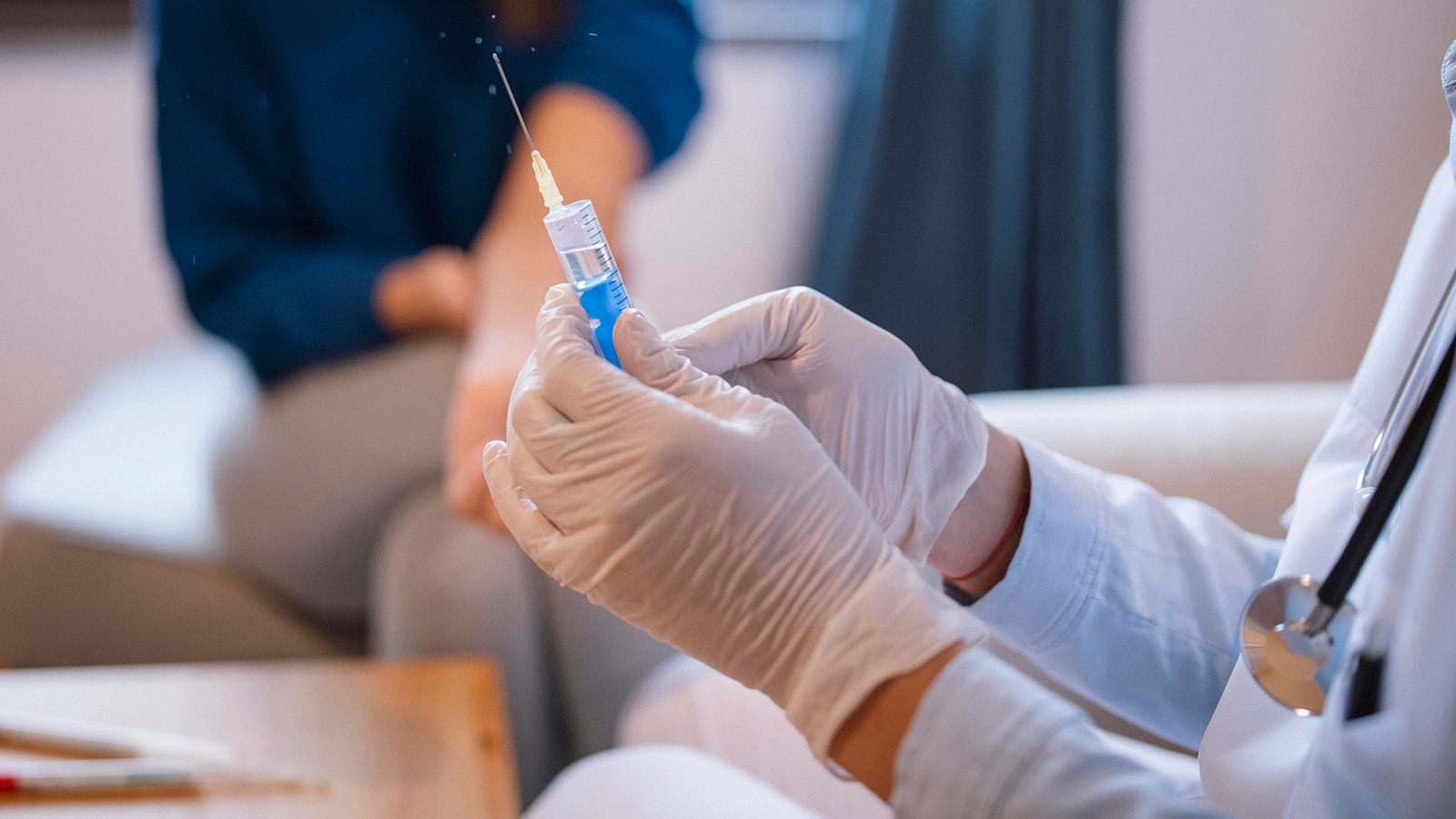
More details have emerged on who will be the first to receive the Pfizer / BioNTech coronavirus vaccine when the UK rollout begins next week, with the first doses now arriving from Belgium.
The long-awaited jab arrived in Britain on Thursday after passing through the Eurotunnel in trucks.
It is now being shipped to each of the decentralized nations and will be distributed to 50 hospital centers in the coming days, and residents and staff of nursing homes and those over 80 will be among the first to be vaccinated.
With the approved vaccine by the UK’s independent health regulator, MHRA, which has defended the speed of its decision following questions from America’s Leading Infectious Disease Expert, the NHS has begun recruiting staff with “experience in delivering a vaccine” or who are “willing to receive training”.
An announcement from a London healthcare trust reads: “We look forward to receiving the Covid-19 vaccine very soon.
“Vaccinating so many Londoners as soon as possible will require your help.
“We are looking for health professionals from across the capital. They may already have experience delivering a vaccine to people or be willing to receive training.”
The government has ordered 40 million doses of the vaccine, which is enough to vaccinate 20 million people, and 800,000 doses are expected to arrive next week.
Studies have shown that it is 95% effective in preventing COVID-19 and works in all age groups.
Chris Hopson, executive director of NHS Providers, said the first clear priority group to receive the Pdo / BioNTech the vaccine would be the residents of the nursing home and their caregivers.
Second, hospitals in the coming days will identify as many patients over 80 as possible who can vaccinate against the coronavirus.
The obvious candidates would be those who attend outpatient appointments and those who receive inpatient treatment.
There were concerns that vaccine storage needs would make delivery to nursing homes difficult, but while the task remains challenging, those fears have lessened somewhat.
The MHRA (Medicines and Health Products Regulatory Agency) has said that boxes of vaccines containing 975 doses can be divided into smaller quantities of doses, making it easier to ship the vaccine to nursing home residents.
Since the vaccine must initially be stored at -70 ° C (-94 ° F), Hopson admitted that “this will not be easy.”
The jab can be shipped to nursing homes as long as it travels no more than six hours after coming out of cold storage, and then placed in a normal refrigerator between 2C and 8C.
If early doses of vaccine remain after these priority groups receive the injection, hospitals will vaccinate staff based on risk factors.
Hospitals will be asked to ensure that anyone who receives a first dose is available for a second dose three weeks later, which is necessary for the vaccine to be effective.
Hopson cautioned that implementation would be a “large, complex and significant logistical challenge,” but added: “This is what the NHS is good at, and why we benefit greatly from having a National Health Service, as creating 33,000 beds Coronavirus patients were shown in the first phase.
“The trusts will deliver this vital task.”
The UK is the first country to approve the use of the jab and the first vaccines are expected on Tuesday.
As more doses become available, possibly in the new year, the vaccine will be given to other risk groups, and most vaccinations are expected to take place between January and April.
There is more of 170 vaccines against coronavirus in development around the world, but there are a handful of pioneers that are in the last stages of verification and could soon be available as the Pfizer jab.
A much cheaper and easier to store option from Oxford University and AstraZenecaAlthough one with a lower efficacy rating, it has been submitted for approval in the UK.
Meanwhile, scientists at the American company Moderna are seeking approval from US and EU regulators to allow emergency use of their jab, which the UK government has approved. ordered seven million doses of.
On Wednesday, Russia announced that it will begin large-scale vaccines using its jab called Sputnik V next week, and the Chinese military approved another made by CanSino Biologics.


