[ad_1]
Clinical trials have started in the UK for a new COVID-19 vaccine that is being developed in Scotland.
The UK government has booked 60 million doses of the candidate Valneva, which is being developed at the French biotech company’s facilities in Livingston, West Lothian.
It is being tested on 150 volunteers at four National Institute for Health Research (NIHR) test sites in Birmingham, Bristol, Newcastle and Southampton.
Commerce Secretary Alok Sharma said he had visited Valneva’s “state-of-the-art facilities” over the summer, adding that he had seen “first-hand the incredible work our scientists and researchers are doing” to develop the new vaccine.
It comes as the UK launches its large-scale vaccination program of the Pfizer / BioNTech vaccine after becoming the first country in the world to approve it on December 2.
Sharma said: “As we take the monumental steps to implement the first COVID-19 vaccine, we must remember that we need to have a range of vaccines available to protect the British public now and in the future.
“Today we have more positive news that life-saving clinical trials will be launched across the country to test the safety and efficacy of the Valneva vaccine, which is being developed clinically right here in the UK.”
Initial trials will show whether the vaccine produces a safe and effective immune response against COVID-19.
If successful, further trials will be planned for April 2021, with more than 4,000 UK volunteers taking two doses, and the candidate could be available by the end of next year.
Valneva will potentially have the capacity to supply up to 250 million doses of vaccines in the UK and around the world.
Company CEO Thomas Lingelbach said: “Our teams have been working extremely hard to develop our differentiated candidate vaccine and I would like to thank them, as well as the UK government, for their dedication and support.
“As we conduct our first clinical trials, we are already increasing our manufacturing capabilities and beginning large-scale production so that we can make the vaccine widely available around the world, assuming the vaccine is safe and effective.”
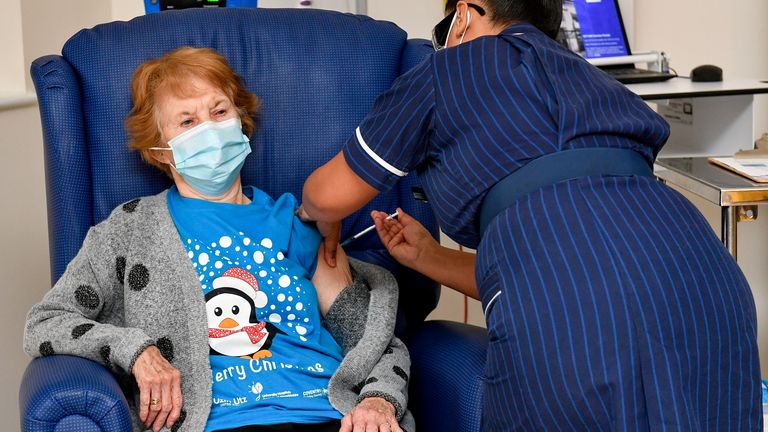
Last week, Margaret Keenan, a grandmother of four, was the first person in the UK to receive the Pfizer vaccine outside of a trial, as people 80 and over and nursing home workers were also among the first to take the hit.
Vaccination centers run by local doctors. in more than 100 locations in England received the jab from Pfizer on Monday in the latest phase of the launch, however some scientists warn it could take in at least one year to inoculate the entire population of the United Kingdom.