[ad_1]
Canada has become the latest country to approve the coronavirus vaccine from Pfizer and BioNTech, describing it as “safe, effective and of good quality.”
In a statement, Health Canada said the authorization was a “critical milestone” in the fight against COVID-19 and noted that the review process had been “rigorous.”
The country is scheduled to receive 249,000 doses this month and four million doses by the end of March.
Overall, Canada has a firm order for 20 million doses of this vaccine with the option to purchase 56 million more, meaning it has purchased more injections per head than any other country.
Officials stressed that “they will closely monitor the safety of the vaccine once it is on the market and will not hesitate to take action if a safety problem is identified.”
Canada currently allows people over 16 to receive the vaccine, but said the rollout could be extended to young people once “clinical trials in children of all ages” are completed.
The announcement comes a week after the UK became the first country in the world to approve Pfizer’s vaccine, which was shown to be 95% effective in preventing disease in large-scale clinical trials.
On Monday, Prime Minister Justin Trudeau said that the first doses of Pfizer’s vaccine will arrive in Canada in late December.
The vaccine will be given free of charge across the country and, like the UK, Canada has said it plans to prioritize the most vulnerable in its society, as well as the doctors and caregivers who care for them.
In an attempt to ease concerns about how quickly the vaccine was approved, Health Canada has released a summary of the evidence that has been reviewed over the past two months.
Scott Moe, who heads the Canadian province of Saskatchewan, said: “We now expect to receive these vaccines faster than originally anticipated and in greater quantities than we had anticipated.”
Canada reported 5,981 new coronavirus cases and 90 deaths on Tuesday. In total, there have been 433,000 infections in the country and 12,931 deaths.
:: Subscribe to the daily podcast on Apple Podcasts, Google Podcasts, Spotify, Spreaker
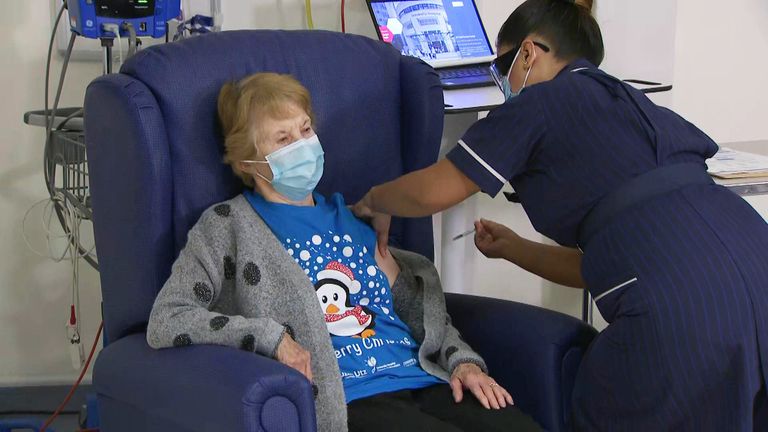
The largest vaccination program in UK history has already been launched, with thousands of people receiving the first dose of the jab yesterday.
Patients require two doses, 21 days apart, to receive full protection against COVID-19.
On Wednesday, UK regulators warned that people who have a history of “significant” allergic reactions you should not get the vaccine.
It came after two NHS staff who were punctured yesterday experienced symptoms of an “anaphylactoid reaction.”
England’s Chief Medical Officer Professor Chris Whitty has said the jab will “substantially” reduce deaths – adding that there will be up to four different vaccines approved for use by the middle of next year.