[ad_1]
The University of Erciyes issued a statement on the vaccine developed against the coronavirus. Although the result of the first dose was shared, the second dose was administered.
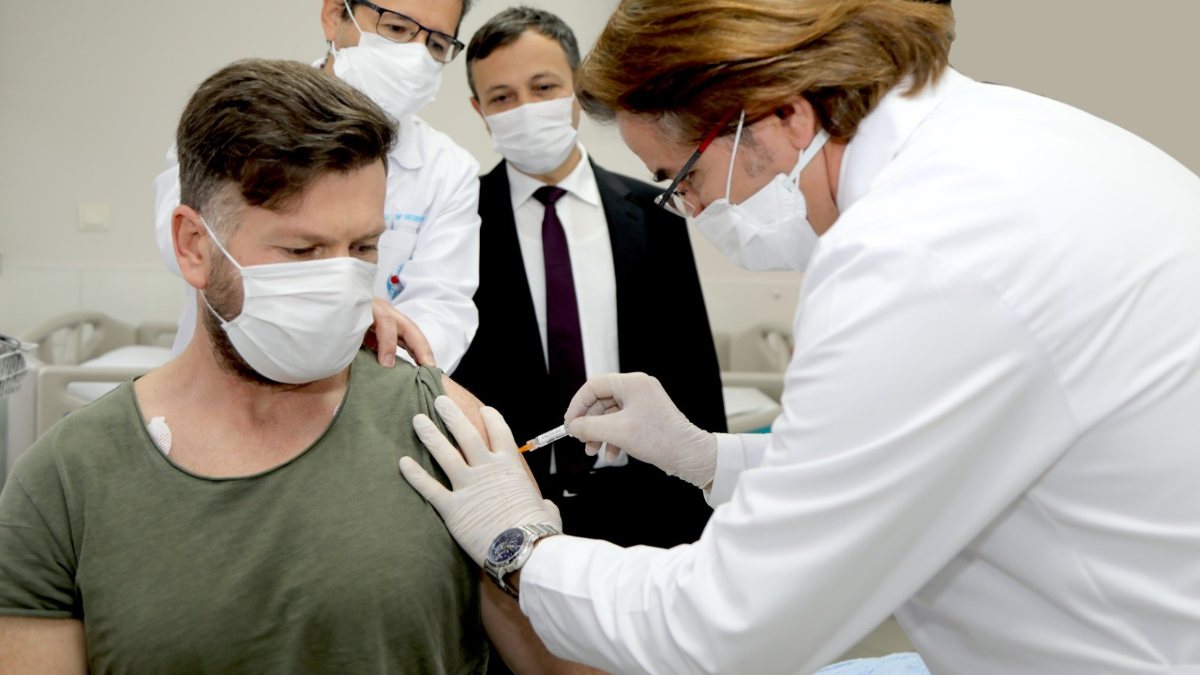
Studies continue on the coronavirus vaccine candidate developed at the University of Erciyes (ERÜ).
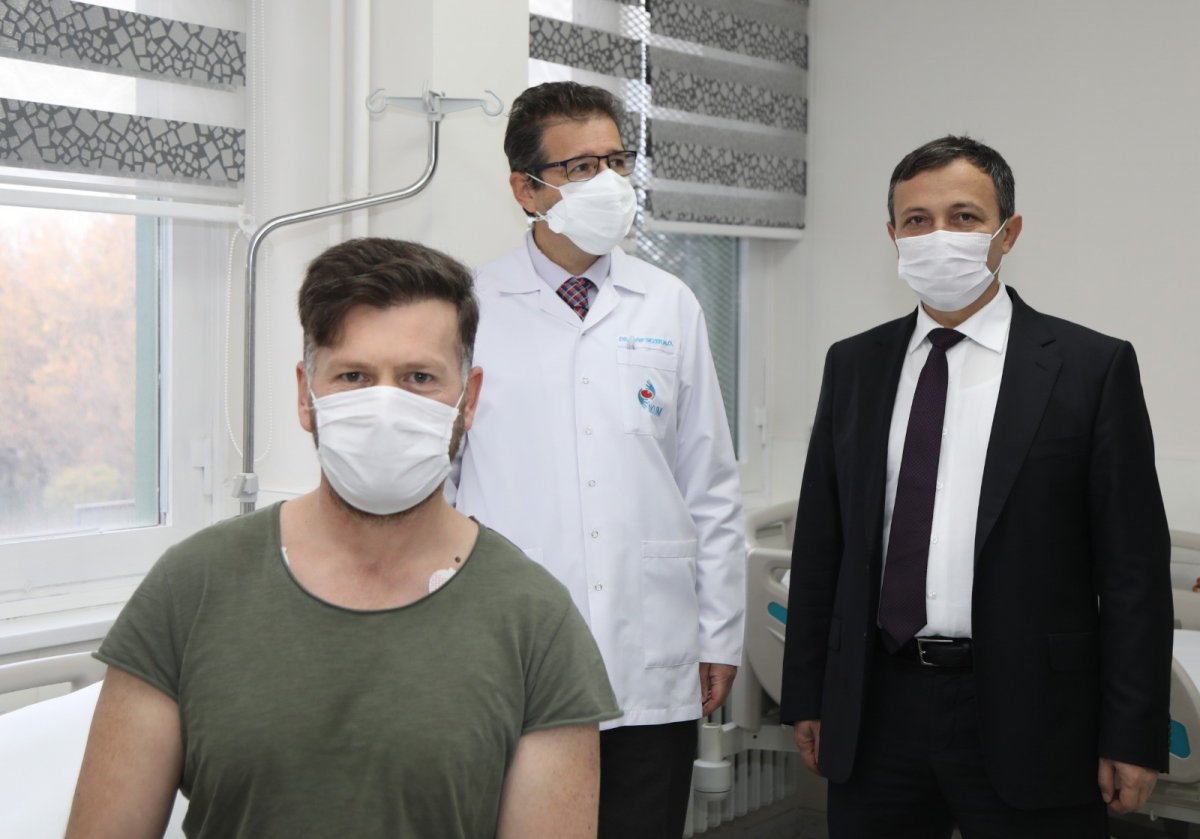
ERÜ Rector Mustafa Çalış spoke about the administration of the second dose of the national and national coronavirus candidate vaccine to the volunteer.
Çalış said that the coronavirus candidate vaccine developed by ERÜ began clinical studies on November 5 and the first dose was completed in 44 volunteers.
Stating that the second dosing started today, Çalış said:
The second dose of the local vaccine candidate was administered with ViDEO
THE SECOND DOSE IS APPLIED
“We also applied the second dose to volunteer Nazif Durna. After the second dose, our volunteer’s condition is very good. 44 volunteers who received the first dose did not have any side effects. Some of our patients had very mild and very little redness. injection site pain This was expected.
OBJECTIVE OF PHASE-2 ON DECEMBER 15
It is very important to have no side effects because phase 1 studies are very important in terms of safety. You must be able to detect security to switch to Phase-2. The first dose was very safe, we hope the second will pass too. As soon as possible, we want to move to Phase 2 on December 15. “
Stating that they are investigating not only the safety part of the vaccine, but also its effectiveness, Çalış emphasized that they hope to switch to Phase 3, such as in late January 2021 or early February, if both safety and effectiveness turn out. very well.

Çalış carried out the following evaluations:
250-350 VOLUNTEERS WILL PARTICIPATE IN THE SECOND STAGE
“I hope that we get to our effective and competent national vaccine as soon as possible. Our president announced, we have the same hope, I believe that our vaccine will be finished in April or spring. We are doing very well so far, I hope that after that, we go very well and we will have our national vaccine in the spring. ” Our 44 volunteers were our volunteers in Phase 1, we will not use them in Phase 2. We are planning to work on at least one volunteer between 250-350 in Phase 2. Volunteers are already applying. Research Center and Good Clinical Practices of the University of Erciyes. what (IKUM) need to apply.

“WE WORK DAY AND NIGHT”
This is one of our Central European drug agencies and is controlled by the many centers in the world of the few rare centers in Turkey. To date, 1,300 phase studies have been carried out at this center. Here everything is done very seriously, we follow the rules. We work day and night to make it fast and of high quality. “
