[ad_1]
Özlem YURTÇU KARABULUT- Buğra BENLİOĞLU- İbrahim MAŞE / TEKİRDAĞ (DHA)
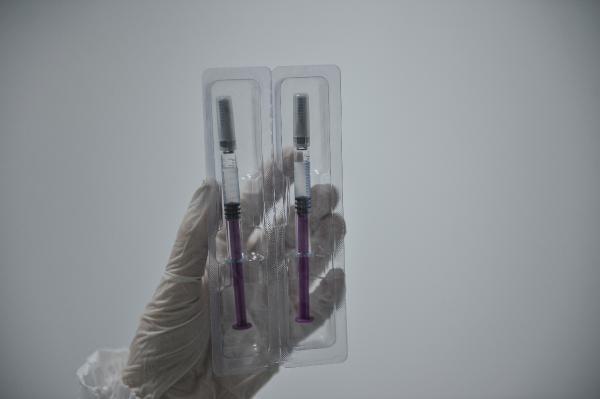
Health Minister Dr. For the phase 1 research Fahrettin Koca gave the good news last week, Koçak Farma’s CEO and CEO Dr. Koçak Farma, who developed the vaccine, will begin to evaluate 44 volunteers later this month. Hakan Koçak shared it for the first time with the Demirören News Agency (DHA). Dr. Koçak said that if all goes well, the vaccine could be launched in May and they have a vaccine production capacity of up to 20 million monthly doses.
5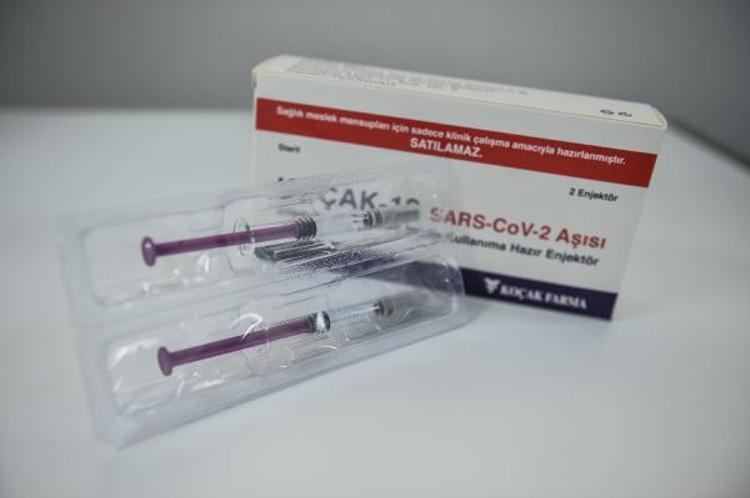
Subscribe

“READY IN MAY, IF EVERYTHING CAME OUT”
Dr. Hakan Koçak said that the Kovid-19 vaccine, which has been studied for months in the R&D department of Koçak Farma, where around 100 scientists work, was designed as a killed virus vaccine known as an inactivated vaccine, ” The characteristic of inactivated vaccines is that it is a technology used in the production of vaccines for many years. Again, the cell lines used here are composed of well-defined cell lines used in previous vaccines … In this sense, inactivated vaccines are vaccines that we can call better known and safer. It can be kept in the refrigerator between 2 and 8 degrees. Therefore, vaccines are easier to protect the cold chain, logistics / transport, and to produce and apply. The world population is around 7.8 billion. So we need a lot of vaccines. For this reason, it is very important that the vaccine is produced quickly and delivered to people. So we must do this as soon as possible. If all goes according to plan, we believe the vaccine will be ready by May. Our vaccine production capacity is 20 million doses per month. Of course, this vaccine will be used mainly for our people in our country. But if our Ministry of Health also has a job in this direction, we have the opportunity to export this vaccine to other countries, “he said.
6
Subscribe

SUCCESSFULLY PASSED THE ‘CHALLENGE’ TEST, NOW ON VOLUNTEERS
Speaking about the results of the vaccine they developed, called preclinical animal experiments, Dr. Koçak provided the following important information about the process that led the vaccine to phase 1 human volunteer trials:
eleven
Subscribe

“At this stage, volunteers are determined by considering risky situations first. For example, since healthcare professionals have a very high risk of contracting Kovid-19 infection, they are given priority in Phase 3. Also, at this stage, while vaccinating a group of volunteers, they are trained a control group giving a placebo to another group. Thousands of volunteers from both Turkey and other countries can also be identified. The important thing here is the cooperation of the countries and their demand for vaccines. In fact, a vaccine developed in China is currently in ongoing phase 3 studies in Turkey. One of the reasons for doing voluntary trials in other countries is that if you consider that this vaccine is used in that country, you evaluate whether the vaccine is also effective in people from that country. If requested, we can conduct voluntary clinical trials in other countries on phase 3 of the vaccine that we have developed. “
12
Subscribe

“OUR PRODUCTION CAPACITY IS HIGH AND WE CAN EXPORT TO THE EXTERNAL MARKET”
When the vaccine is available to meet the needs of the first targets in Turkey is as Dr. Hakan Kocak emphasizes, Turkey about 3 years ago, including Switzerland, USA, UK, Ireland, Germany, Canada, Japan and Australia , which also included the “International Drug Control Association Emphasizing that it was accepted as a member of (PIC / S) ö, said:” Member countries here can recognize each other’s licenses and inspections on drugs or vaccines. This Membership will play an important role in the export of domestic vaccines. Apart from that, to export vaccines abroad, you must prepare a file containing the results of your study and send it to authorities such as EMA in the European Union and FDA in the USA. “If your file is reviewed and found to be appropriate, your vaccine export opportunity is strengthened,” he said.
twenty-one
Subscribe

General Manager and CEO of Koçak Farma, Dr. Hakan Kocak …


