[ad_1]
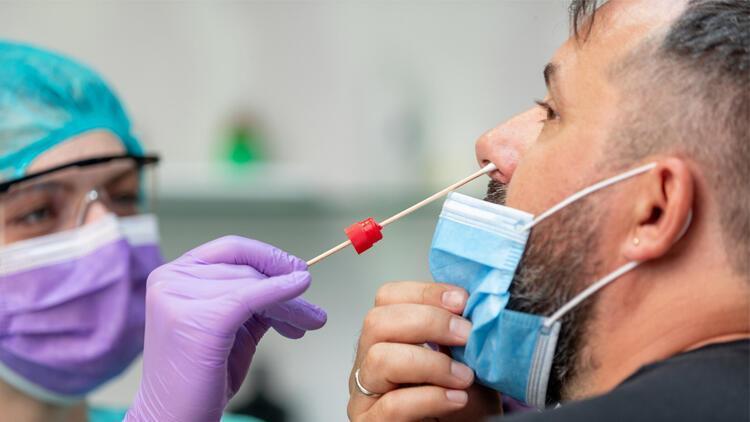
According to information obtained by the AA reporter, the Health Ministry took action after it was determined that the Kovid-19 diagnostic kits, the sale of which is prohibited on the Internet, were sold on social media and Internet shopping sites.
Ministry of Sales due to the Presidency of Turkish Pharmaceutical Products and Medical Devices Agency Kovid-19 test kits were examined for notifications made in connection with advertising and promotional activities.
ACCESS DENIED
During the epidemic process, it was observed that 39 of the 90 notifications made to the Ministry regarding the sales, advertising and promotional activities of the Kovid-19 test kits contained complaints about the sales and advertising activities of the Kovid test kit. -19 made on social networks. After examining the activities determined to be carried out on social networks, the issue was referred to the relevant companies and access to the links was blocked.
WRITTEN WARNING
class = “cf”>
On review, 28 of the notifications were found to include sales of Kovid-19 test kits and website advertising. In this context, the website address with a built in Turkey, a written warning that the termination of these activities.
While 20 of the notifications were found to be related to product sales made through hosting providers (shopping sites), notifications were sent to relevant hosting websites regarding incompatibilities.
It was determined that the 3 notifications included sales made directly by phone and WhatsApp messages.
Likewise, the Provincial Health Directorates corresponding to the corresponding Provincial Health Directorates were notified to carry out the necessary inspections in accordance with the legislation on companies with legal personality whose sales addresses and advertising activity were determined, and inspections were carried out in topics directly covered by market surveillance and inspection.
According to the legislation, devices that are intended to be used and applied by health professionals or that require their application in a medical device sales center cannot be advertised to the consumer. Kovid-19 diagnostic kits are also considered in this context.