[ad_1]
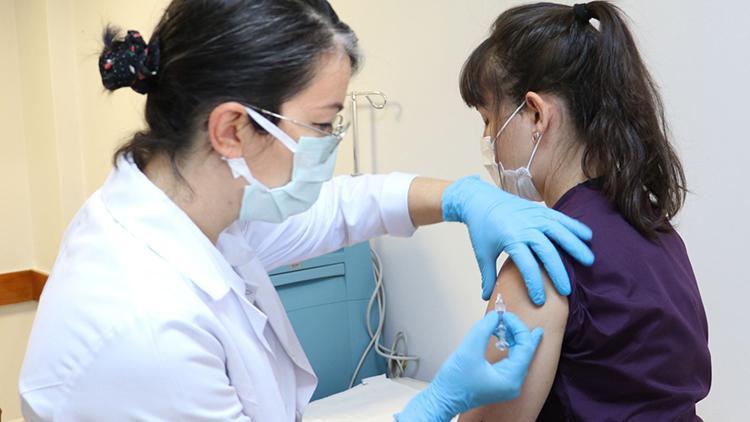
Hacettepe University School of Medicine, Department of Infectious Diseases and Clinical Microbiology, Professor. Dr. Murat Akova evaluated DHA on the first voluntary application of the Covid-19 vaccine developed in China. Prof. Dr. Akova said that the vaccination studies began on Thursday last week, they preferred vaccination in the high-risk group, so they gave priority to health workers.
Prof. Dr. Akova recalled that they will first vaccinate 1,200 volunteer health workers, “These vaccinations will also be made in 24 other centers besides us. Hacettepe is the first coordinating center that started vaccination. We plan to start working in one or more centers in Istanbul this week. Then we plan to start working on other centers step by step. ” We will start working. These centers are currently the ones that have not yet begun to study the main reason why a bureaucratic team operates. The main lead sponsor of the Turkish Ministry of Health Institutes of Health Chair of this study. Therefore, there is a brokerage firm that they appoint. An equipment contract is signed with the hospital through intermediaries. There have been some delays, but I think it will be resolved within this week, “he said.
‘WE DID NOT EXPERIENCE VERY SERIOUS SIDE EFFECTS’
class = “cf”>
Prof. Dr. Murat Akova stated that they conducted various tests on the volunteers who requested vaccination and said: “We do not want to vaccinate a large number of volunteers at the same time. To ensure easy control and provide a more comfortable service to these people. We have vaccinated to around 20 volunteers so far, all of them health workers. ” Before vaccinating these people, we draw blood from them and check for antibodies. At the same time, we check for viruses, even if they have no symptoms. 2 of the volunteers who applied us tested positive for the antibody. So they had the disease without symptoms. Therefore, we were unable to vaccinate these 2 people. But the rest of the volunteers received the first dose of vaccines. Fortunately, we did not experience any serious side effects in the last 5 days. There are minor things, of course, like mild fatigue, malaise, pain where the shot was given. “No problem for now.”
‘WE LISTED THE VOLUNTEER REQUESTS’
class = “cf”>
Prof. Dr. Akova recalled that they have not yet received any official request from volunteers other than health professionals, “but our citizens who are aware tell us that they want to volunteer and we make a list of their demands. But since we have not officially opened this application, the number is not very high. “We will try to accept volunteers once we finish it. As soon as we decide to do the vaccine in a wide scope, we will go back to them and say ‘we can inoculate’, “he said.
‘IT WILL LAST UP TO 7 MONTHS’
Prof. Dr. Emphasizing that the vaccine will be administered to the volunteers in two doses, Murat Akova said:
“After 14 days, we will give another dose of the same vaccine to the people who received the first vaccine. On days 28 and 42, we will draw blood from these people and look for antibodies in their blood. In addition, we will periodically test these people with the samples which we call PCR to detect the presence of viruses.no, but is there any immunity that develops in the body other than the antibody that we call ‘T-cell immunity’ for some of them, which is supposed to be more durable. if available. These patients will be monitored, examined, monitored by phone periodically. We will be notified immediately. We will do a safety analysis. It is quite a long process, it will take up to 7 months. Maybe we will follow up later. We are at the beginning and it’s too early to say anything. “
‘WE DO NOT HAVE A VACCINE WITH TOTAL SAFETY’
class = “cf”>
Prof. Dr. Murat Akova had the following to say about when the widespread use of the vaccine, whose voluntary practice began, will begin:
“If approved, it will be used outside of us. This vaccine has now been approved for emergency use by the Chinese authorities. We know that it also applies to military and medical personnel in China, but we have no information on how many people it was applied to. . According to one information, side effects of fever, headache and fatigue were observed in 4 patients. We have not received safety data yet. We have not found serious and serious side effects. All these are scientific studies. There is no such vaccine. There is vaccines that have received a rate of urgent use in Russia and China by the authorities there. This is also criticized by the scientific world. Because although they have given such approvals, the effectiveness and reliability of these vaccines have not been scientifically proven in large masses.
‘WE WILL DECIDE THE EFFECTIVENESS OF THE VACCINE’
Prof. Stating that the number of volunteers will increase in the coming days, Akova said: “We are giving a vaccine to a group and a group of Covid vaccines. Although we do not want these volunteers, we will analyze this data if symptoms appear, we will see if it was in the vaccine or it was in the vaccine. ” Therefore, we will decide the effectiveness of the vaccine, but until those results are given, it cannot be said that we have a safe vaccine that should definitely be used, it is scientific research, we are currently continuing it.
‘WE ARE EARLY BELOW THE LOCAL’
Prof. Dr. Murat Akova, while the domestic vaccine studies addressed was still in the initial phase, “than in animal studies still. Preceding the various phases of man. A total of 9 vaccines registered in the list of the World Organization of the Health, but I know about 13 vaccines administered in different centers in Turkey. These are one or “There is information that some may have reached the human trial stage,” he said.

