[ad_1]

Source, fake images
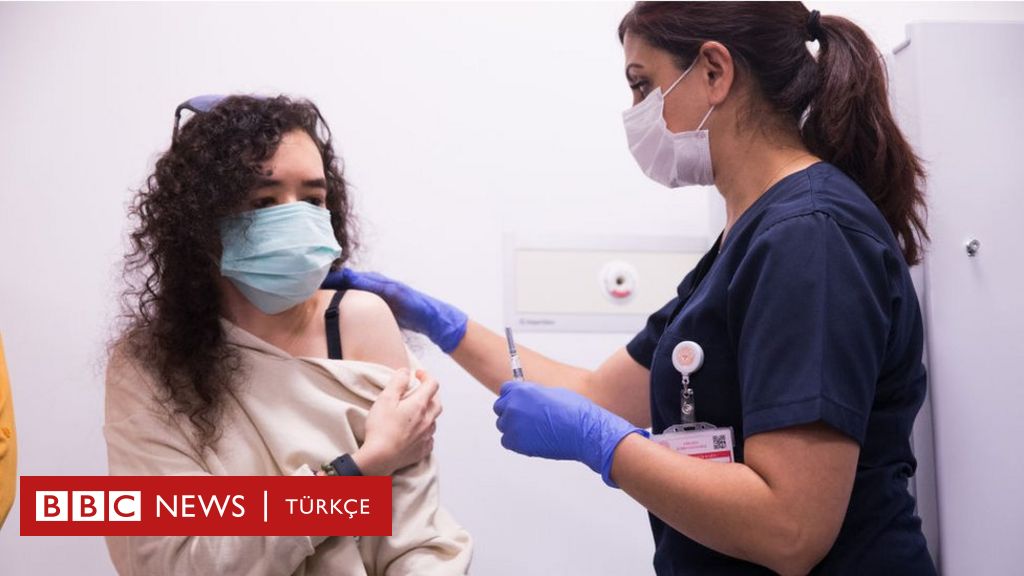
A volunteer administered the Covid-19 vaccine produced by the China-based company Sinovac in Ankara.
The Minister of Health, Fahrettin Koca, announced today the Coronavac vaccine developed by the Chinese company Sinovac at a press conference held in conjunction with the Scientific Committee on Sunday night to go to Turkey.
Member of the Scientific Committee Prof. Dr. Sedat Ünal announced that the effectiveness rate of the vaccine was 91.25 percent.
The Scientific Committee reviewed data from 1,322 people who received the second dose of the vaccine in two weeks.
Among them, 3 cases of coronavirus were found in 752 people who were vaccinated, while there were 26 cases of coronavirus in 570 people who received a placebo.
Based on these data, the Scientific Committee announced the effectiveness of the vaccine (1-RR) at 91.25 percent. The confidence interval (CI95) is specified between 71 and 97 percent. This is data that shows how much protection the vaccine can provide when given to larger groups, as it has been tested in a small number of people.
Member of the Scientific Committee Prof. Ünal stated that the vaccine provided 71 percent in the worst case and 97 percent in the best case and added:
“91 percent will increase in the next few days, we are almost certain of it. Because initially the number of people we gave a placebo to was less than the number of people we vaccinated. As the number of patients increases in that group, it will increase the efficiency of 91 percent. “
The world’s first data
This statement was the world’s first information on Coronavac.
Health Minister Koca said that the Chinese state will also use this data.
According to the Scientific Committee statement, 6 of the cases in the placebo arm required serious treatment with hospitalization.
After vaccination, 2 out of 3 people who had a case of coronavirus had no symptoms, while one person only had a runny nose.
Side effects
The most common side effects after the first dose of vaccine are explained below:
- Fatigue: 4.7 percent
- Headache: 3.9 percent
- Pain at the vaccination site: 1.9 percent
These side effects were claimed to be seen at lower rates after the second dose.
Prof. Unal said: “One of our friends had an allergic reaction and was treated. Other than that, no serious side effects were observed,” he said.
Husband Ministers 9 million people in the first phase planning to vaccinate, the vaccine is expected to reach 50 million by the end of February in Turkey, the possibility of vaccinating up to 2 million per day in Turkey has announced that it has been found.
In addition to family doctors, hospitals will also be used for vaccination activities.
Source, Reuters
Coronavac developed by Sinovac
Who will get vaccinated first?
Vaccination will be carried out in Turkey in four separate groups.
The first group includes healthcare workers, people over 65, people with chronic diseases, those living in nursing homes and nursing homes, and the disabled.
In the second stage, police, soldiers, teachers, judicial officials and prison staff will be vaccinated.
The third group includes employees in the service sector and the fourth group includes other people.
Health Minister Koca said at today’s press conference: “Our goal is to vaccinate people in the third stage no later than April, such as January, February and March.”
Source, it gave
What happened to the Pfizer BioNTech vaccine?
Health Minister Fahrettin Koca said he had many phone calls with BioNTech owner Uğur Şahin about purchasing the vaccine from Pfizer and BioNTech, and that he was asked to add a non-liability clause for the production of the vaccine, and that the contract could not be signed until today because they did not accept it.
The husband, after 5-6 phone calls with Uğur Şahin in the last 2-3 days, agreed to a clause stating that the contract was responsible for the amount of the contract, the contract would be signed in a few days, and at the end of March , 4.5 million doses of vaccine, if necessary, 30 million doses. announced that it would be taken.
How was the data evaluated?
Health Minister Koca explained the data analysis process as follows:
“To start the vaccination program as soon as our Turkish Pharmaceutical Products and Medical Devices organization (TİİCK) was asked to transmit the intermediate results from the centers conducting clinical trials.
“These data, which will form the basis for the approval of emergency use, were evaluated by the independent evaluation committee and transmitted to TİİCK. Our institution presented the provisional results of the study to the Scientific Committee. We are confident in the effect of our vaccine on our own people, with the evaluations of our Scientific Committee.
How much does the vaccine cost?
Minister Koca said the prices determined in the contract they signed with both companies for the vaccines should be kept secret, but they bought it at a lower price than is sold to many countries.
The husband, when asked by a journalist, cited the delay in the vaccines that were planned to arrive last week, as well as the prolongation of the permit processes.