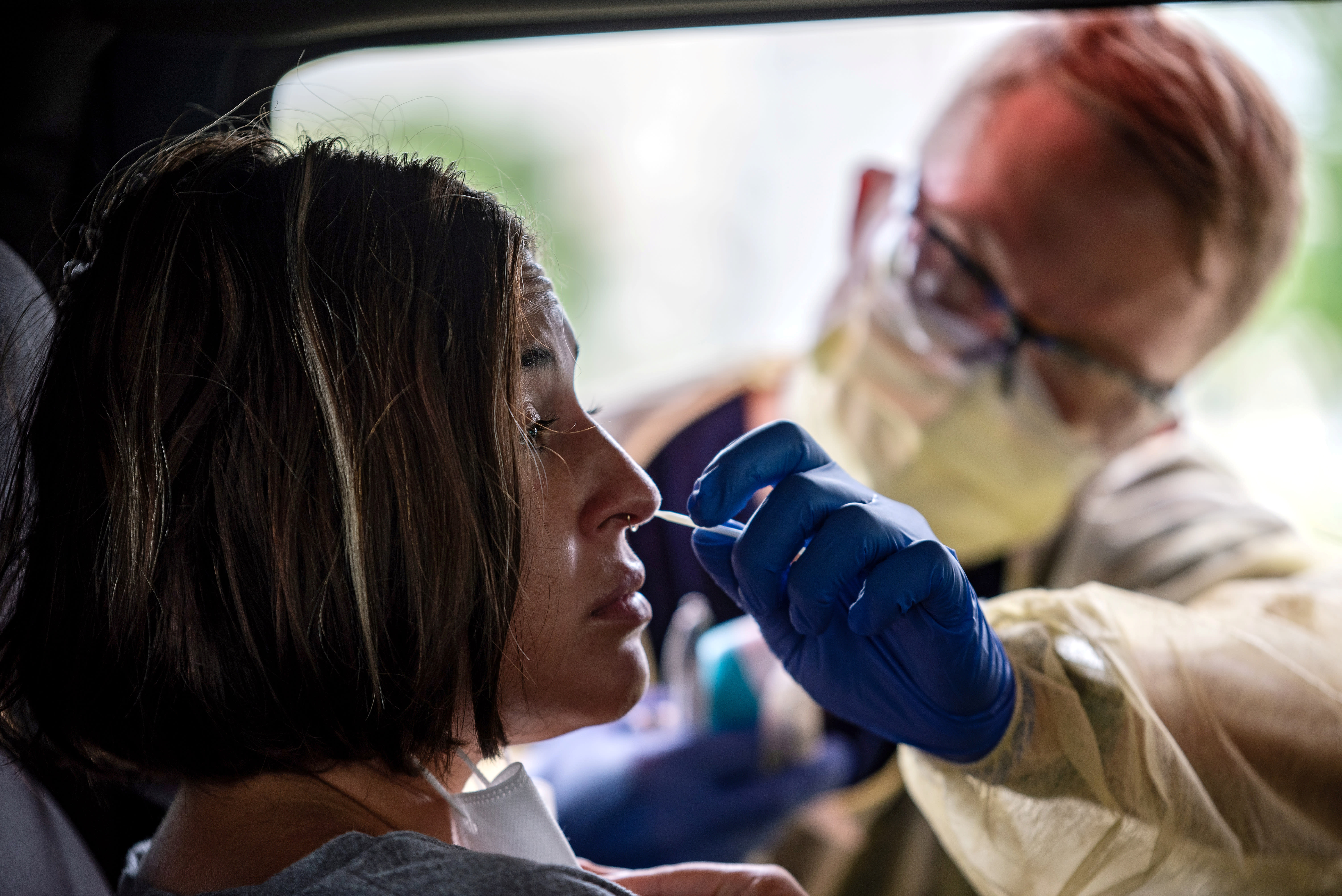
A case confirmed in Nevada on Friday has changed the fear of loneliness in Hong Kong as the possibility of retrieval of Covid-19 patients for the recovery of a case confirmed in Nevada announced on Friday has created new and worrying wrinkles.
The Nevada man first tested positive for the virus in April, and was confirmed again 48 days later in June. Some experts have said that patients can stay positive for months without symptoms and recovery. However, the latest developments confirm that even those who recover are not protected indefinitely by antibodies.
The researchers confirmed Nevada reinfection by genetic data in two positive results, which were different. The case is extending concerns about the second wave coming in the fall and winter – set to coincide with the annual flu season.
The global epidemic has reached about 24.5 million cases and affected more than 832,000 people. The US, India and Brazil top the charts with millions of cases, while Russia is close to one million cases. More than 6 million domestic cases are being closed, and more than 1, 000 people have died.

U.S. There are fewer cases per week than week. (Graphic: David Foster / Yahoo Finance)
Countries around the world continue to fight global epidemics as large companies receive treatment and vaccines at unprecedented speeds.
U.S., U.K. And while Western countries like Germany are working on frontline vaccine candidates, Russia claims to be ready to use the vaccine already, and China is issuing emergency use authorization to several candidates.
According to reports, Synovac’s candidate, Coronavac, was authorized last month, and SynoPharm’s candidate was authorized recently. But China has not officially given many details.
Last month, the country authorized the use of trial vaccines on high-risk individuals, and the candidate for cancino has been authorized for use by the military for one year.
According to the Van Street Journal, Cancino is looking outside China, as the Canadian-Chinese company seeks emergency approval for its candidate from other countries.
While the seeds of nationalism have been sown in the race for vaccines, with countries questioning whether they can get vaccines, it remains to be seen who signals with China.
Whether other countries will trust the regulatory and safety process in China is a matter of concern, as pharmaceutical companies Or make it easier to communicate freely and openly with China’s regulatory bodies than in Europe.
Zac Emanuel, vice provost for global initiatives at the University of Pennsylvania and chairman of Medical Ethics and Health Policy, said the race to be first is not an ideal strategy.
“Both China and Russia and the UK see this as a test of their science and capabilities around the finish line. I keep saying … just before the finish line shows how easy it is to make samples, how effective they can be, “Emanuel told Yahoo Finance.” There are likely to be many versions of the coronavirus vaccine first. Round durability and effectiveness will be poor.
When asked if the U.S. If the Chinese can use vaccines, it is not necessary to consider, said Anthony F. C., director of the National Institute of Allergy and Infectious Diseases. “My prediction would be that we will have more than one candidate who will ultimately prove safe and effective,” he said in a recent interview with Yahoo Finance.
@ Anjkhem“data-reactid =” 46 “>Anjali Khellani a Journalist On Yahoo Finance. Follow her on Twitter: @ Anjkhem
More from Anjali:
Twitter,Facebook,Instagram,Flipboard,SmartNews,LinkedIn,YouTube.“data-reactid =” 53 “>Follow Yahoo Finance on Twitter, Facebook, Instagram, Flipboard, SmartNews, LinkedIn, YouTube.